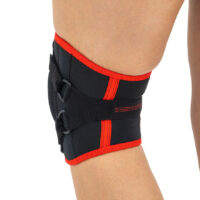
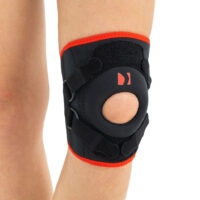
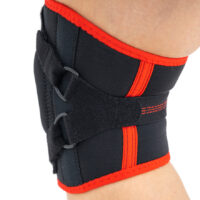
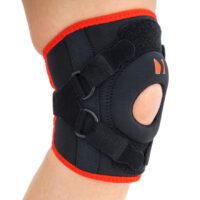
Knee orthosis AM-OSK-Z
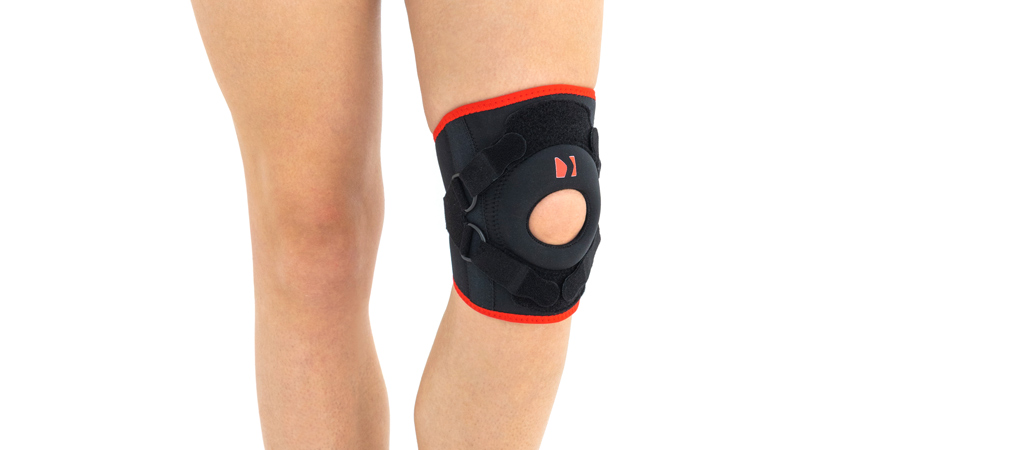

 Knee brace
Knee brace Anatomic patella donut
Anatomic patella donut Class I medical device
Class I medical device Compression
Compression Silicone pad
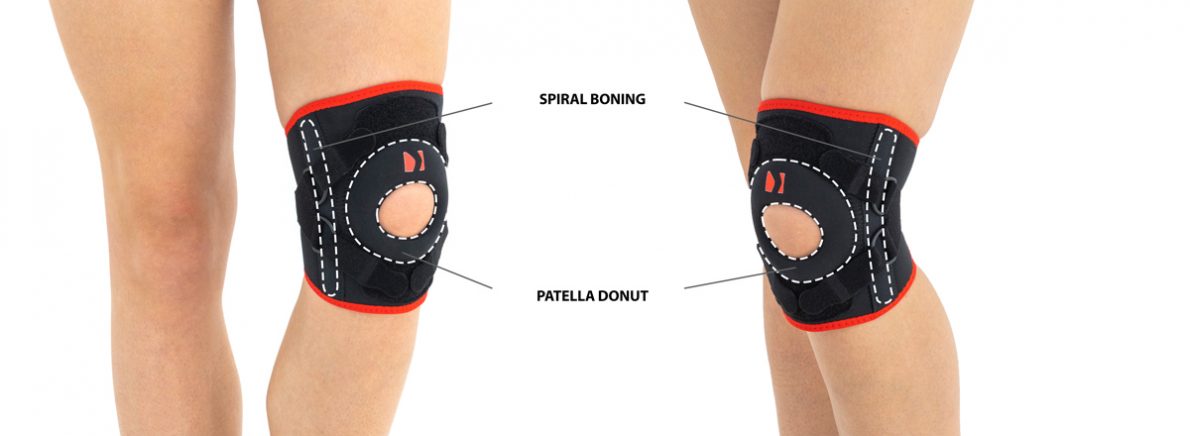
Silicone pad Spiral bonings
Spiral boningsPATELLA KNEE BRACE
Description
Patellar injuries
The sudden injures involve patella dislocation and fracture. The patella dislocation is the result of the ligaments weakening and it’s connected with haemorrhage. The kneecap moves usually to the knee lateral side. The fracture might be caused by direct blow (e.g. kicking into knee or fall onto knee) or indirectly by sudden contraction of quadriceps muscle. This kind of injuries occur in every sports and can lead to chronic patellofemoral instability.
Our patella knee brace AM-OSK-Z is highly recommended to prevent against the patella dislocation.
Product description
Kneecap support AM-OSK-Z with patella donut is made of innovative and skin-friendly material, called ActivePren™ and AirRubber III™.
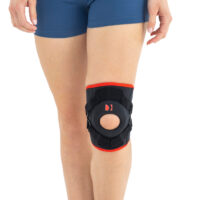
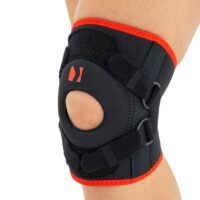
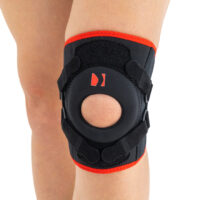
Our kneecap support AM-OSK-Z supports and stabilises injured, weak or arthritic kneecap and patellofemoral joint during occupational and sporting activities. It’s equipped with anatomic-shaped patella donut and special rear and front straps providing excellent patella stabilisation. Polyamide buckles allow to adjust the level of knee compression.
Special front donut prevents against the mal-tracking of patella and helps with patellofemoral strains, sprains and instability.
Purpose of use
AM-OSK-Z brace should be applied in case of:
- patellofemoral instability
- patella dislocations
- kneecap fractures
- chondromalacia patella
- arthritic pain
Sizes
| Size | Knee circumference | How to measure |
| S | 30-34 cm (11,8″-13,4″) |
 |
| M | 34-38 cm (13,6″-15″) |
|
| L | 38-42 cm (15,2″-16,5″) |
|
| XL | 42-46 cm (16,7″-18,1″) |
|
| XXL | 46-50 cm (18,3″-19,7″) |
Total length of the product: 19 cm (7,5″)
Gallery
Technology
MATERIALS
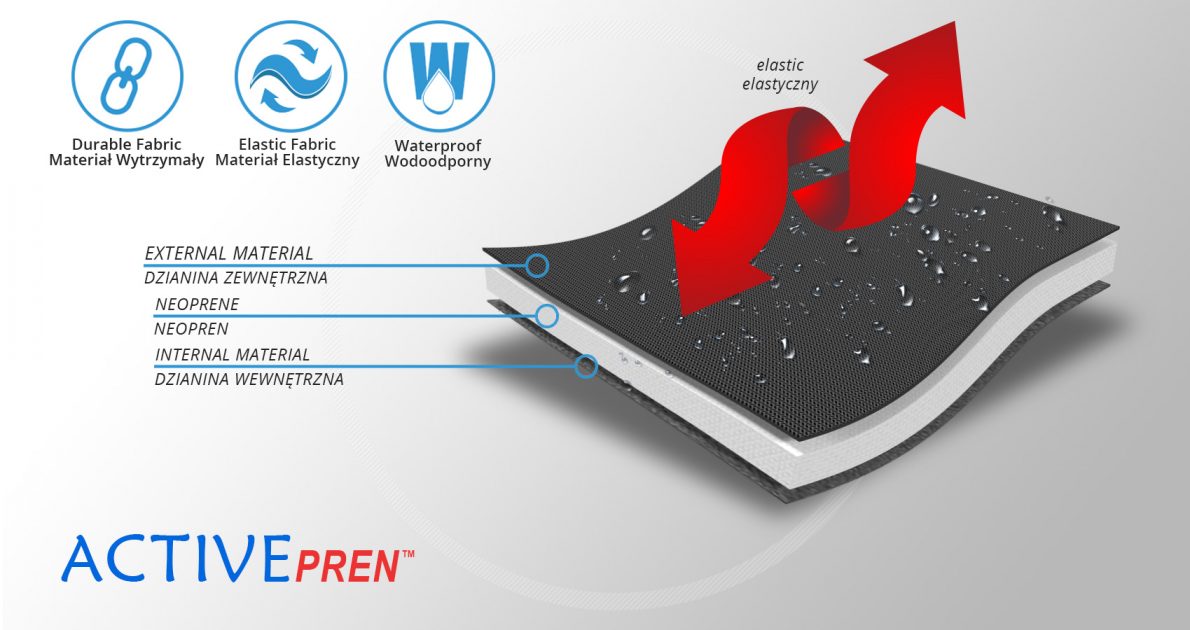
ActivePren™
ActivePren™ is an active three-layer material consisting of two elastic jersey cover fabrics and a core made of neoprene foam. This material is characterized by softness and high flexibility. A very important advantage of this material is the fact that it is not a knitted product, it does not have thick fibers, so that the weaves of the material do not imprint on the patient's skin and do not cause abrasionsin places of high compression. Products made of ActivePren are the strongest and most effective stabilizing orthoses available on the market.
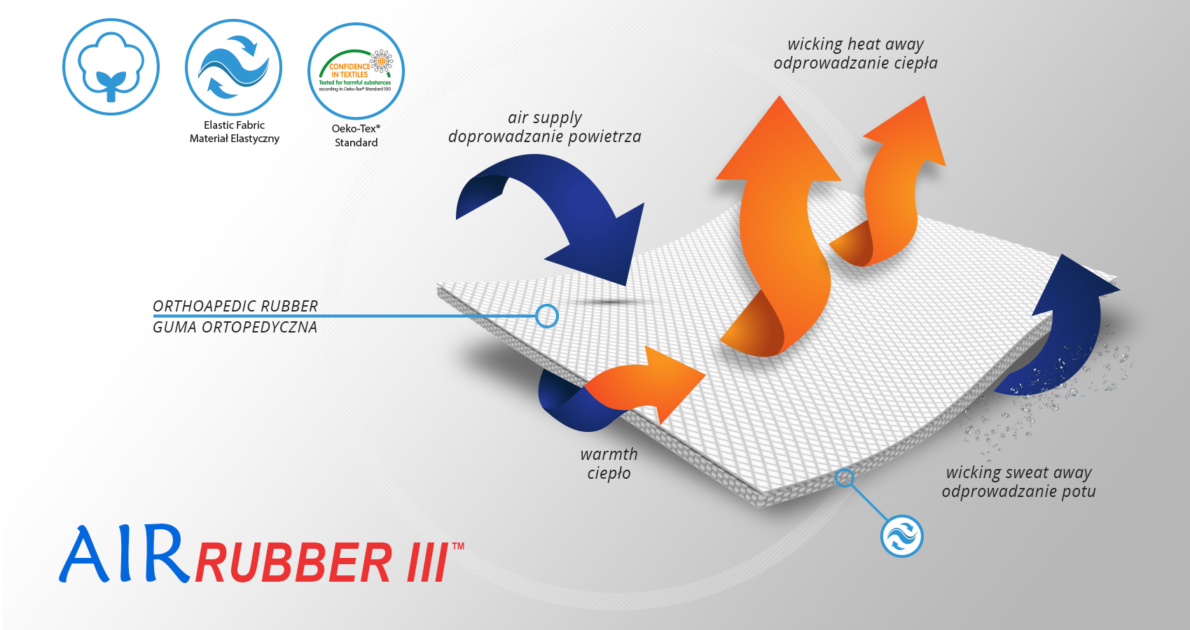
AirRubber III™
AirRubber III™ has unidirectional elasticity. It can be stretched, increasing the length, not width, what improves compression. Between the braids air flows freely and skin can breathe. In addition in high compression, these rubber braids provide a gentle massage for skin. Our orthopedic rubber is very friendly for skin.
STIFFENINGS
Spiral boning
Our orthopedic spiral boning is a thin wire, coiled into a spring and flattened. We use boning of various widths and wire thicknesses, which determines the strength of their stabilization. Each spiral boning has two specially profiled fittings to prevent damage of the orthosis. They are galvanically protected against corrosion by the galvanizing process, so they are resistant to water, moisture and sweat. Products equipped with bonings can be washed without removing them from the orthosis. They work in every direction, perfectly adjusting to the user's body and have a shape memory function, thanks to which they always return to their original profile. This function causes the spiral boning in the orthosis to stabilize the swollen limb immediately after the injury and after the swelling has come off.

PADDINGS
Patella stabilizers
Relief stabilizers of various shapes made in 2D technology. They are made of relieving foams connected on one side with a fabric friendly to the patient's skin, and on the other with a gripper, thanks to which the stabilizer can be attached to the adhesive element of the orthosis. Thanks to such a structure, these pads do not have to be sewn into the orthosis, and they can simply be fastened to it from the inside. These elements have an anatomical shape and are made of comfortable foam with proper hardness and elasticity, which guarantees the proper therapeutic effect.
Setting up
Downloads
ON OUR WEBSITE WE PRESENT MEDICAL DEVICES.
USE THEM ACCORDING TO THE INSTRUCTIONS FOR USE OR LABEL.

 Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
MANUFACTURER / ADVERTISING ENTITY: REH4MAT Sp. z o.o.