PVN – INCONTINENCE VISMEMO™ COVER
The outer layer of the cover is made of elastic PureMed™ material – its washable properties are an ideal solution while using the BodyMap® cushions at home, rehabilitation centers or health care facilities, where cleanliness and hygiene are of vital importance. This is especially important when more than one person is using the BodyMap® product.
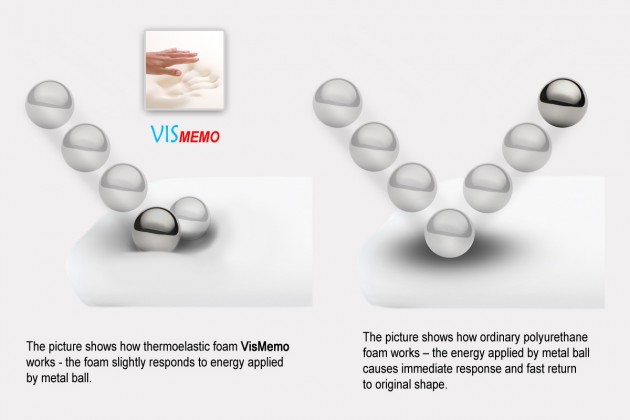
The second layer of the cover is the VISmemo™ memory foam which adjusts its shape to the shape of the user’s body under pressure. The VISmemo™ is most active in the areas most prone to bedsores, i.e. where the pressure is the highest.

The stabilizing and positioning BodyMap® system is made of an innovative grey fabric PureMed™ which has numerous advantages:
- It is safe for people and very easy to disinfect;
- Ideal for use in areas with the high risk of infection;
- There is a possibility of multiple use, it does not rub off;
- It is perfect when the cushions are used by different users, e.g. in hospitals or rehabilitation centers;
- It is lightweight, soft, elastic and easy to shape;
- Contains antibacterial components based on inorganic Silver Zeolite;
- It meets all the essential requirements of the medical products of different kinds*;
- It has OEKO-TEX® STANDARD 100 certification which is granted to exceptionally skin-friendly parameters;
- It is used as a cover for hospital mattresses, operating tables and couches;
- It is waterproof and vapour-permeable in one way only which means that the air from the inside of the cushion may migrate freely outside through the PureMed™ cover.
- At the same time, liquids such as sweat, blood or urine will not be able to get through the cover inside the cushion;
- The PureMed™ cover has passed the CIGARETTE TEST and the MATCHSTICK TEST positively, which proves that it is a non-flammable fabric;
- Complies with REACH regulation aimed at ensuring a high level of health and environmental protection.
* According to the Regulation of the Ministers of Health on 3 November 2004 . and the requirements contained in Annex I to Council Directive 93/42 / EEC of 14 June 1993 concerning medical devices , deployed by the law of 30 April 2004 of Medical Devices ( OJ L 93 of 2004 . , pos. 896 ).
This is a medical device. Use it in accordance with the user manual or the label.

