Hip support AM-SB/1RE
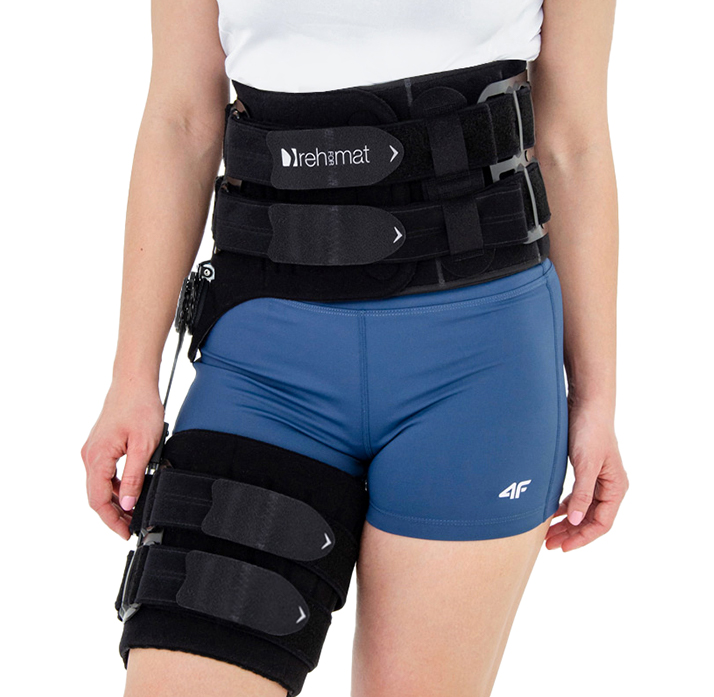

 Hip brace
Hip brace Class I medical device
Class I medical device Dial joint 1RE
Dial joint 1RE Full range of adjustment
Full range of adjustment Innovative
Innovative Latex-free
Latex-free Press-slide
Press-slide Recommended by specialists
Recommended by specialists Universal size
Universal sizeVIPER
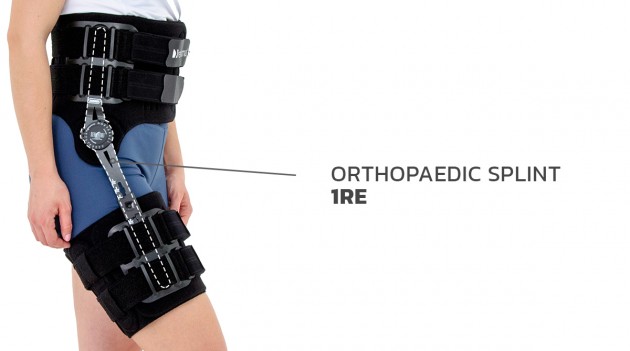
HIP ORTHOSIS WITH SPLINT 1RE
Description
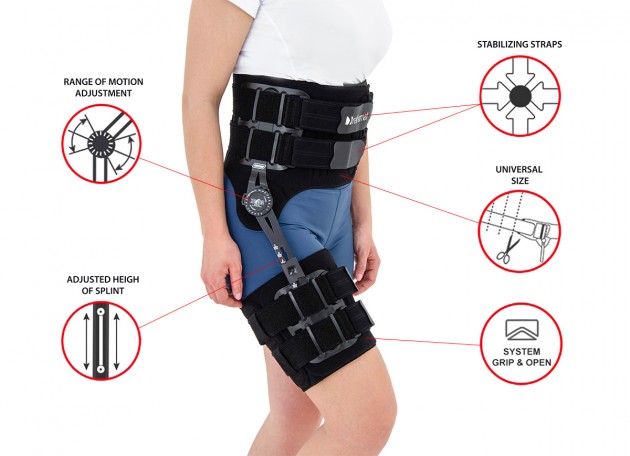
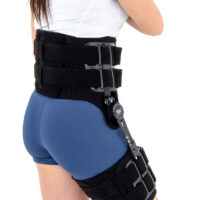
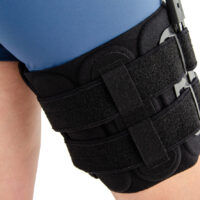
Immobilizes or controls the pelvis joint, equipped with pelvis frame, thigh band and lateral splint 1RE with adjustment of the flection and extension angle every 15 degrees. The brace assures correct positioning of the pelvis during upright standing and learning to walk, prevents and helps in treatment of contractions. Use: in early rehabilitation, during learning to walk after surgery treatment of the pelvis joint, in treatment of patients with contractions of pelvis joint due to neurological or orthopedic problems.
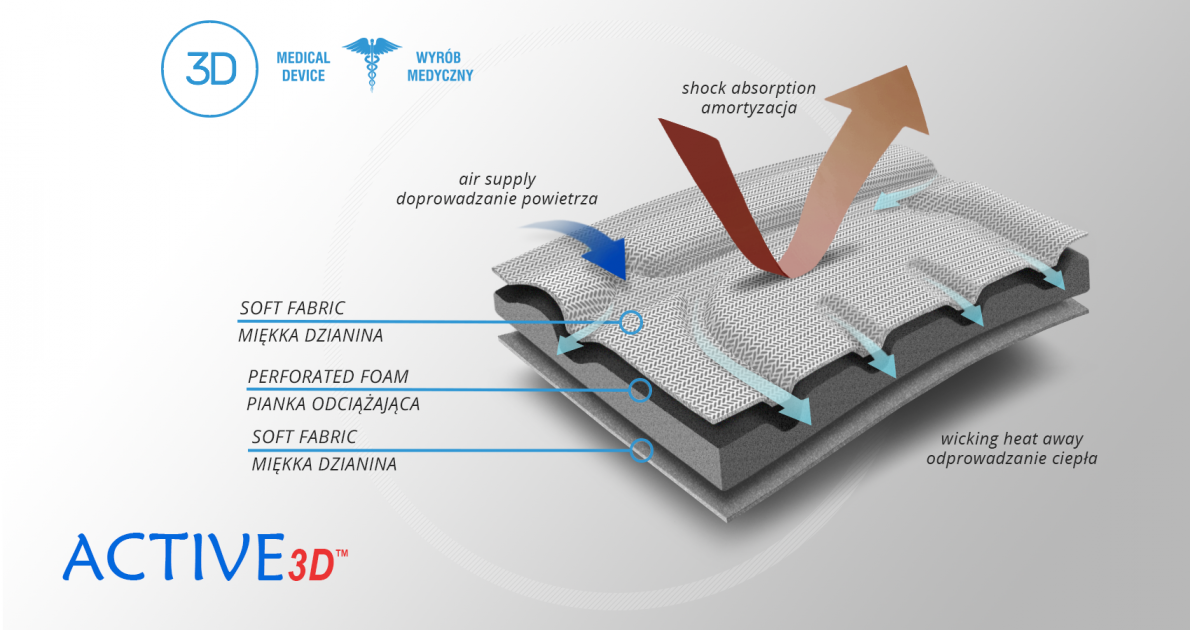
The brace is made of Active3D™.
The element of the lap belt which is used to adjust the circumference made of ActiveDistance™.
Properties
– the support assures correct positioning of the hip during upright standing and learning to walk. It prevents contractures and helps healing them. It enables controlled movement of the hip joint. Instruction of use is given by the leading doctor.
Purpose of use
– In early rehabilitation and during learing towalk in case of patients after the hip surgery treatment
– In rehabilitation of patients with contracture of the hip joint due to the neurological or orthopaedic reasons
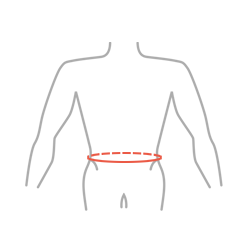
Sizes
| Size | Waist circumference | How to measure |
|---|---|---|
| UNIVERSAL | 75 – 125 cm (29,5″ – 49,2″) |
 |
Left/right available
Maximum user weight up to 75kg.
Total length of product:
min: 53 cm (20,9″)
max: 67 cm (26,4″)
Gallery
Technology
MATERIALS
Active3D™
Active3D™ is thermoformed fabric made of special foamed, cell-closed designed material. It reduces the pressure on the body or any abrasions made by orthopaedic stays and aluminum splints. It is fully waterproof fabric and does not absorb sweat. It’s easy to clean. Due to its features, the fabric is an excellent product for making medical orthopaedic braces and orthoses. ACTIVE 3D™ has various external self-gripping layers. Our material has special, thermoformed properties and may be shaped according to the functional goals of the final braces.
ActiveDistance™
ActiveDistance™ is an technical off-loading lamination with 3-layered construction. It’s made of EVA foam double-sided laminated with non-elastic polyamide layer that is easy to attach. This self-gripping function of ActiveDistance™ provides wide range of adjustment and perfect fitting. What is important, ActiveDistance™ is non-elastic fabric so it stabilizes your body in a perfect way. Thanks to using the comfort foam, the device is really soft what influences on the comfort of using it. Waterproof material.
TECHNOLOGICAL SYSTEMS
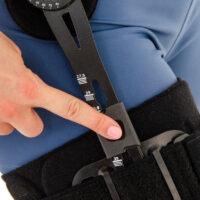
Press-slide System – Regulation of the length of splint

Press-slide system is the another technological newness in our offer that we would like to introduce to You with a great pleasure. The adjuster of the length of splint Press-slide is the element of orthopaedic braces, designed especially according to the innovative rule – MiniMax (minimum choice, maximum effect). That is why, orthopaedic devices equipped with Press-slide system may be characterized by small dimensions, very low weight and significant ergonomics of usage. What is more, the system was engineered to facilitate the extension or the shortening of the splint to the patient in the most easy and safe way. Press-slide was made of special kind of plastic strengthened by fiberglass and thanks to that solution its durability is much more noticeable than in case of aluminium. Adjustment of splints is very easy – you just need to press the special button and ajustd the splint so that it would be fully comfortable for you. To make that activity easier, on the surface of the lower limb splints we placed the centimeter scale that defines the distance between knee joint and the end of thigh splint and shin splint. Regarding upper limb braces, we placed the scale that simplifies the repeatable splint adjustment.

STIFFENINGS
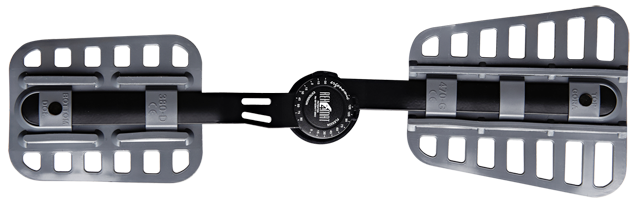
Splint 1RE
Splints 1RE – single axis orthopedic splints, used mainly in knee joint, upper limb and hip joint braces. The characteristic quality of this splint is, apart from wide range of motion adjustment in every 15 degrees, is also hyperextension adjustment in the secured range of -15 and -30 degrees. The splints are characterized by perfect lateral joint stabilization and low weight. The set-up of the angle adjustment clock doesn’t require any tools, and special safety system prevents the change of the angle by unauthorized people. The splints are made of high quality aluminium alloy and plastic with carbon fibre. They are light and neutral to the influence of sweat and salt, properly firm, assuring correct functioning of the device. The splint and its design are patented in European Union by Reh4Mat company.
PADDINGS
3D supports
3D relief supports are independent technical solutions to relieve the rigid elements of a given orthosis. These elements are made of supporting foams or EVA foam. These foams are connected with various types of skin-friendly materials and materials with an adhesive function. These pads have the appropriate shape and color adapted to the type of orthosis. They relieve both metal elements of orthoses, such as splints, stays, underwires and orthopedic drop locks, as well as other elements that should not come into direct contact with the patient's skin. These pads have an anatomical shape and are made of comfortable foam with proper hardness and elasticity, guaranteeing the proper therapeutic effect.
Downloads
ON OUR WEBSITE WE PRESENT MEDICAL DEVICES.
USE THEM ACCORDING TO THE INSTRUCTIONS FOR USE OR LABEL.

 Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
MANUFACTURER / ADVERTISING ENTITY: REH4MAT Sp. z o.o.