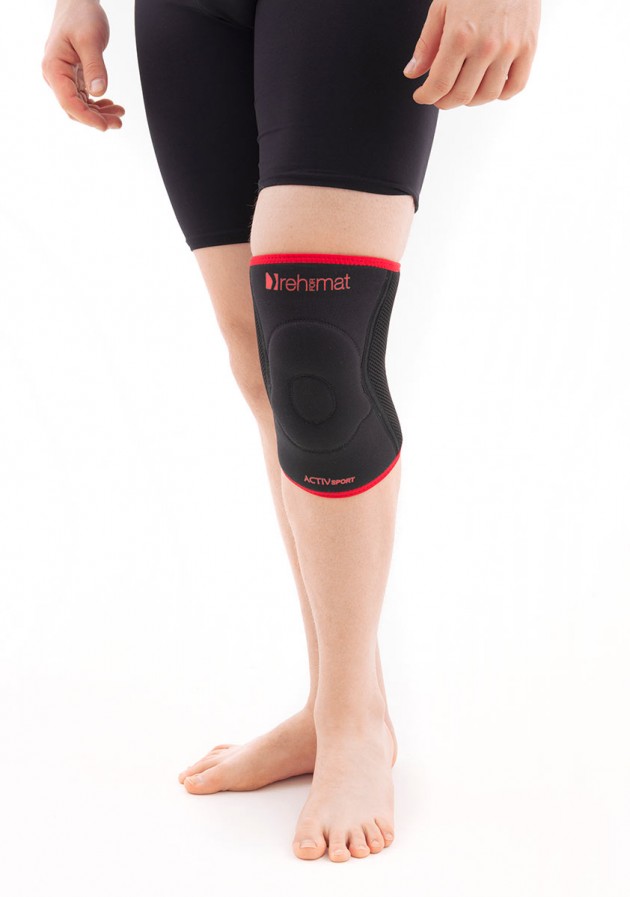
Knee orthosis AS-SK/F


 Knee brace
Knee brace Class I medical device
Class I medical device Compression
Compression Latex-free
Latex-free Silicone pad
Silicone pad Skin-friendly
Skin-friendly Spiral bonings
Spiral boningsANATOMIC KNEE BRACE WITH ORTHOPAEDIC STAYS AND SILICONE PATELLA SUPPORT
Description
The product is available while stocks last
Product description

Knee support AS-SK belongs to the PROTECT BY SILICONE group. It is active brace to knee support and stabilize, in case of its instability, swelling or pain. The brace stabilizes joint, relieves pain and increases mobility.
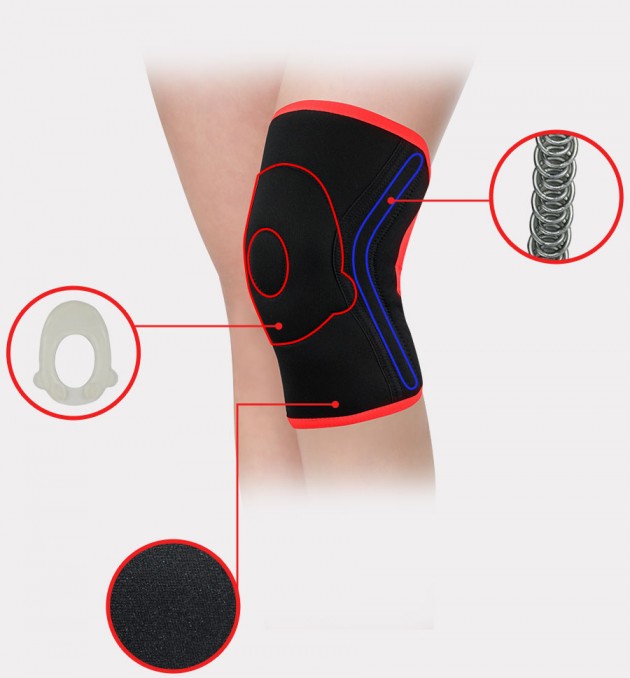
The brace is equipped with silicone patella donut what sets new standards in orthopaedic supply.
The brace is equipped with sides spiral stays, which stabilize knee joint additionally, what allows to use it in sport trainings.
Knee support AS-SK belongs to the PROTECT BY SILICONE group.
The brace is made of innovative, providing compression and skin-friendly fabric called ActivePren™ and ActiveSpace™.
Purpose of use
AS-SK/F brace should be applied in cases of:
- knee dislocation,
- knee twist and sprain,
- little side instability of the knee joint,
- little kneecap instability
- prophylactically in sport
Sizes
Available sizes
| Size | Knee circumference | How to measure |
| S | 30-34 cm (11,8″-13,4″) |
 |
| M | 34-38 cm (13,6″-15″) |
|
| L | 38-42 cm (15,2″-16,5″) |
|
| XL | 42-46 cm (16,7″-18,1″) |
|
| XXL | 46-50 cm (18,3″-19,7″) |
|
| XXXL | 50-54 cm (19,9″-21,3″) |
Technology
MATERIALS
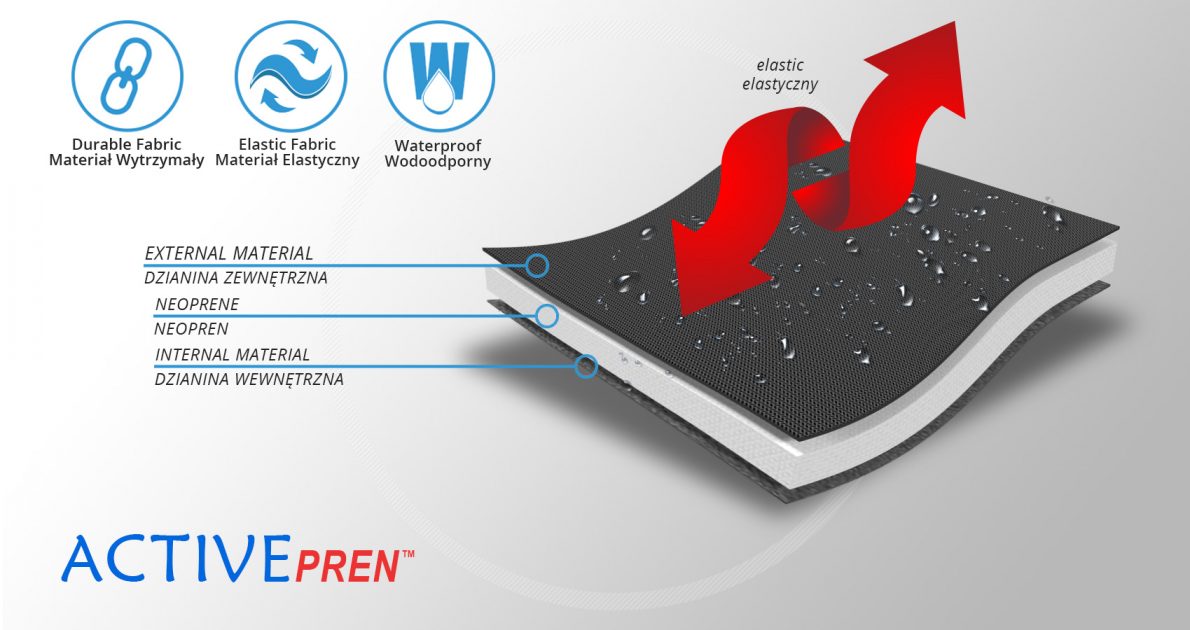
ActivePren™
ActivePren™ is an active three-layer material consisting of two elastic jersey cover fabrics and a core made of neoprene foam. This material is characterized by softness and high flexibility. A very important advantage of this material is the fact that it is not a knitted product, it does not have thick fibers, so that the weaves of the material do not imprint on the patient's skin and do not cause abrasionsin places of high compression. Products made of ActivePren are the strongest and most effective stabilizing orthoses available on the market.
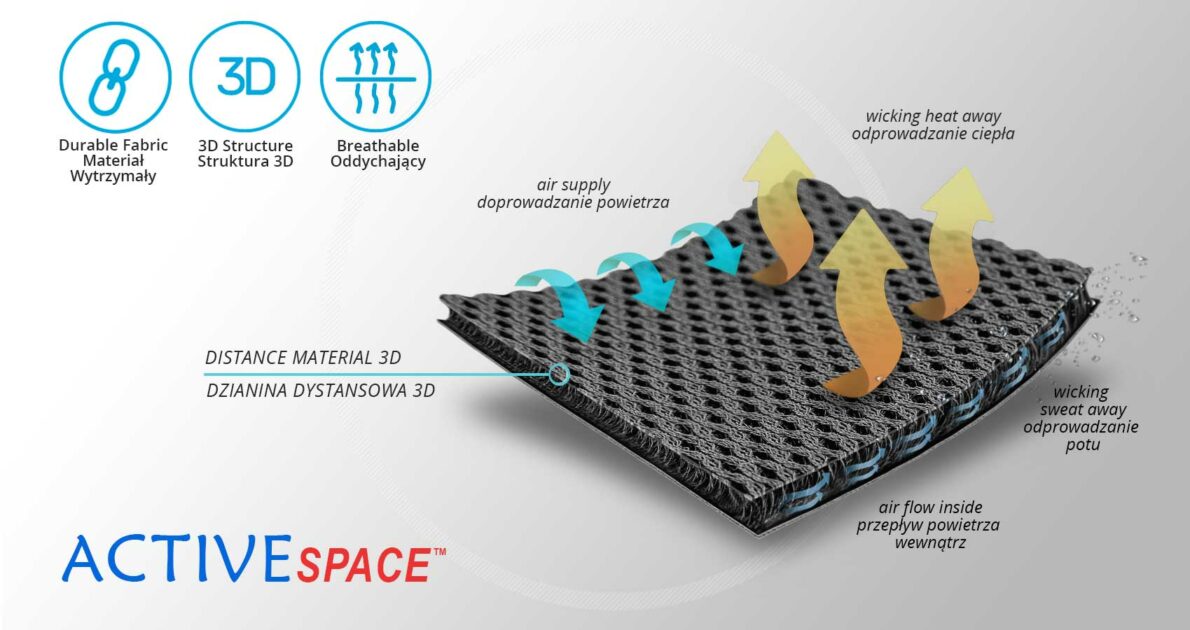
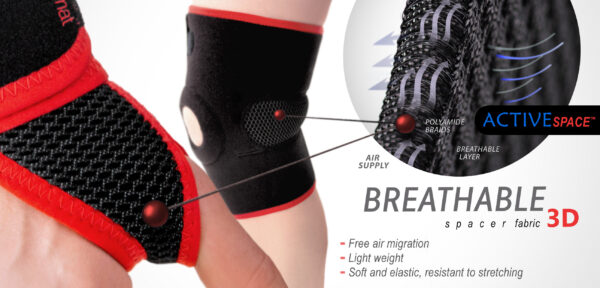
ActiveSpace™
ActiveSpace™ is a spacer, polyamide 3D lamination with high skin ventilation. It is very lightweight, consisted of 2 layers. Between them, we use polyamide braids. ActiveSpace™ is not elastic what improves stabilization. Inside the lamination, between 2 layers, the air flows freely, maintaining minimal water and moisture absorption. Waterproof material.
TECHNOLOGICAL SYSTEMS
PROTECT BY SILICONE
![]()
Setting up
Downloads
ON OUR WEBSITE WE PRESENT MEDICAL DEVICES.
USE THEM ACCORDING TO THE INSTRUCTIONS FOR USE OR LABEL.

 Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
MANUFACTURER / ADVERTISING ENTITY: REH4MAT Sp. z o.o.