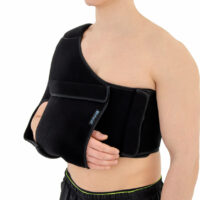
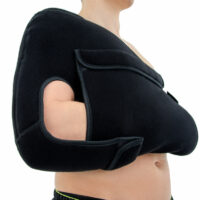
Upper limb support AM-BX-02


 Upper limb brace
Upper limb brace Breathable
Breathable Cast replacement
Cast replacement Class I medical device
Class I medical device Double-sided
Double-sided Durable
Durable ER
ER Orthopedics
Orthopedics Recommended by specialists
Recommended by specialistsPOSTOPERATIVE SHOULDER AND ARM IMMOBILIZER, DESAULT
Description

Purpose of use
Should be applied in cases of:
– dislocations (as temporary protection) in shoulder joint,
– severe pain complexes in shoulder joint,
– injuries of soft tissues of trunk,
– operations of shoulder joint,
– necessity of supporting and partial immobilizing of upper limb,
– after taking off the plaster dressing as stabilizer of shoulder.
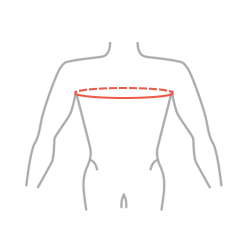
Sizes
| Size | Chest circumference | How to measure |
| S | to 75 cm (to 29,5″) |
 |
| M | to 85 cm (to 33,5″) |
|
| L | to 95 cm (to 37,4″) |
|
| XL | to 105 cm (to 41,3″) |
|
| XXL | to 120 cm (to 47,2″) |
|
| XXXL | to 135 cm (to 53,1″) |
Gallery
Technology
MATERIALS
ActiveDistance™
ActiveDistance™ is an technical off-loading lamination with 3-layered construction. It’s made of EVA foam double-sided laminated with non-elastic polyamide layer that is easy to attach. This self-gripping function of ActiveDistance™ provides wide range of adjustment and perfect fitting. What is important, ActiveDistance™ is non-elastic fabric so it stabilizes your body in a perfect way. Thanks to using the comfort foam, the device is really soft what influences on the comfort of using it. Waterproof material.
Setting up
Downloads
ON OUR WEBSITE WE PRESENT MEDICAL DEVICES.
USE THEM ACCORDING TO THE INSTRUCTIONS FOR USE OR LABEL.

 Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
MANUFACTURER / ADVERTISING ENTITY: REH4MAT Sp. z o.o.