Torso support AR-SO-02


 LSO
LSO Breathable
Breathable Class I medical device
Class I medical device Compression
Compression Efficiency
Efficiency Orthopedics
Orthopedics Recommended by specialists
Recommended by specialistsOPENWORK LUMBAR BACK SUPPORT WITH ELASTIC STRAPS
Description
The orthosis AR-SO-02 is characterized by strong circumferential stabilization on lumbar area, thanks to elastic fasteners.
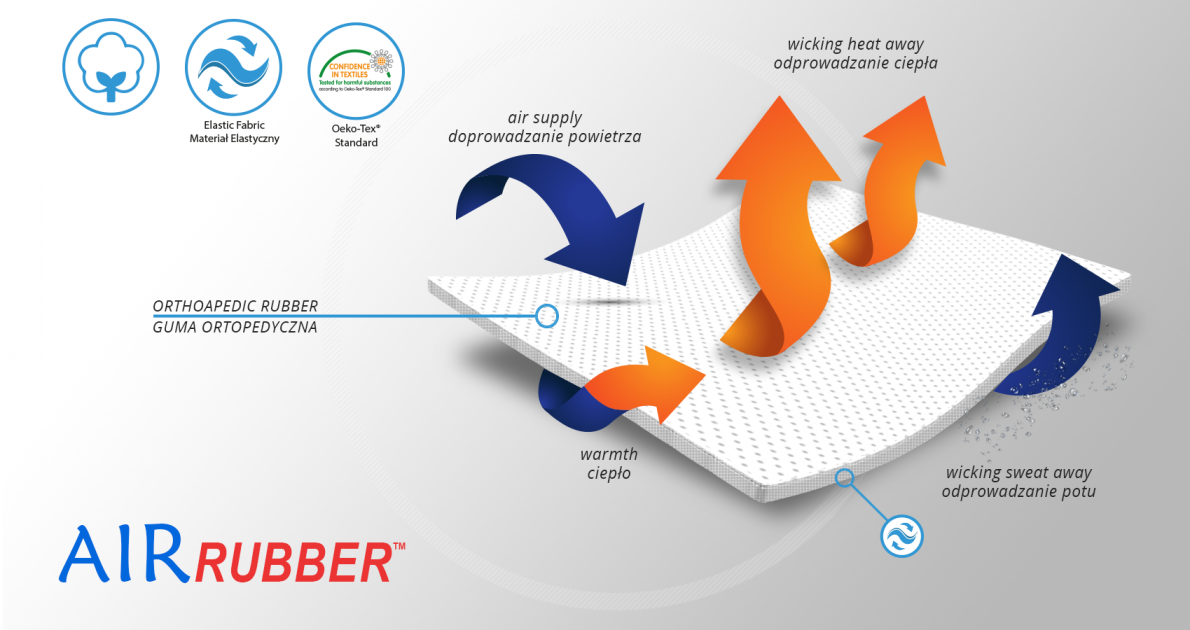
The orthosis is made of strong orthopaedic rubber AirRubber™.
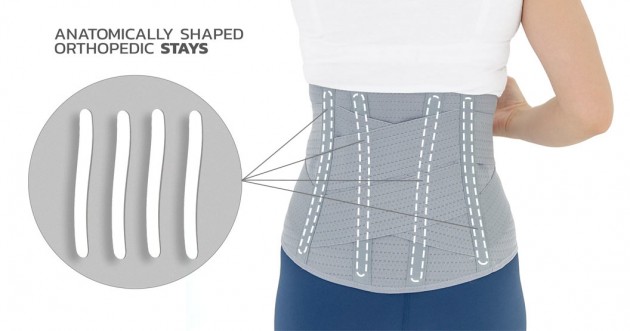
The Orthosis is provided with anatomically shaped metal stays (sweat-resistant coating), which quantity depends on the size; 4 or 6 metal stays in the Back. The Cross System in the back provides additional reinforcement of stabilization at Lumbar area.
Properties
– SKIN FRIENDLY PRODUCT – lumber support has been made of special orthopaedic rubber, containing mainly cotton, which is neutral to patient’s skin
– EFFECTIVENSS OF STABILIZATION – thanks the anatomically shaped orthopaedic nibs and tightening rubber belts crossed at the back, the stabilization is exceptionally good.
– SAFETY – the front securing tape provides additional protection and the chances of the device to undo accidentally are eliminated.
– COMFORT OF USAGE – the limber support is very light and therefore comfortable to wear.
Indications
– Pre- and Post-Surgical Stabilization
– Degenerative Spinal pathologies on Lumbar area
– Disc Hernia
– Lumbar Muscles and Ligamentous Strains and Sprains
– Postural Support
– Chronic Low Back Pain
– Rehabilitation and Prevention
Sizes
| Size | Waist circumference | How to measure |
| S | 65-75 cm (25,6″-29,5″) |
 |
| M | 75-85 cm (29,5″-33,5″) |
|
| L | 85-97 cm (33,5″-38,2″) |
|
| XL | 97-110 cm (38,2″-43,3″) |
|
| XXL | 110-125 cm (43,3″-49,2″) |
|
| XXXL | 125-145 cm (49,2″-57,1″) |
S , XXXL – last items
Total height of the product:
front: 17 cm (6,7″)
rear: 29 cm (11,4″)
Gallery
Technology
MATERIALS
AirRubber™
AirRubber™ has unidirectional elasticity and it’s breathable. This lamination is perforated. Between the braids air flows freely and skin can breathe. In addition in high compression, these rubber braids provide a gentle massage for skin. Our orthopedic rubber is very friendly for skin.
STIFFENINGS
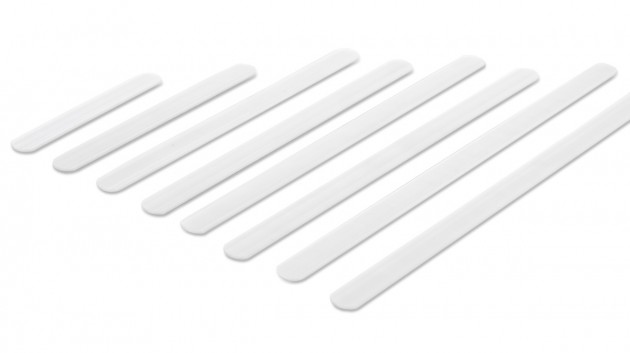
Orthopedic profiled stays
Orthopedic stays are made of special spring steel, covered with a plastic coating and have rounded and protected ends. They can come in various widths and thicknesses as well as different hardness. They can be pre-shaped or flat. They work in one direction and adapt to the shape of the body while stabilizing the laterally protected part of the body. They are perfectly protected against corrosion, so they are resistant to water, moisture and sweat. Products equipped with them can be washed without removing them from the orthosis. They adjust to the body. The orthopedic stays cannot bend and that is why, they cannot correct the body posture or the secured joint.

Plastic stays
They come in various widths and thicknesses, are made of various types of plastics, such as polyamide, ABS or acrylic, and these features determine their stiffness. Thanks to their design, they are resistant to water, moisture and sweat. Products equipped with them can be washed without having to remove them from the orthosis. Our plastic stays work only in one direction, perfectly stabilize the laterally protected part of the body, adjusting to it at the same time and have a memory function, thanks to which they always return to their original shape. This function causes the stays in the orthosis to stabilize the swollen limb immediately after the injury and after the swelling has come off. The plastic stays cannot bend and that is why, they cannot correct the body posture or the secured joint.
Setting up
Downloads
Accessories
ACCESSORIES / PRODUCTS TO BE USED WITH
ON OUR WEBSITE WE PRESENT MEDICAL DEVICES.
USE THEM ACCORDING TO THE INSTRUCTIONS FOR USE OR LABEL.

 Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
MANUFACTURER / ADVERTISING ENTITY: REH4MAT Sp. z o.o.