Torso support AM-TX-02

 Chest belt
Chest belt Class I medical device
Class I medical device Invisible
Invisible Recommended by specialists
Recommended by specialists Skin-friendly
Skin-friendly Universal size
Universal sizeUniversal Thorax belt
Description
Surgeries on the chest aren’t pleasure for patients. Apart of side effects such as pain or immobilization, the fear of the breathing is the most serious. The patient with the chest injury feels the pain and it’s afraid of breathing because of possible of cough can torn the po-op injury. The patients breath shallowly so the fluid around the lung is the result. The deep breath is very important because it improves the lungs, brain and other organs blood circulation and oxygenation.
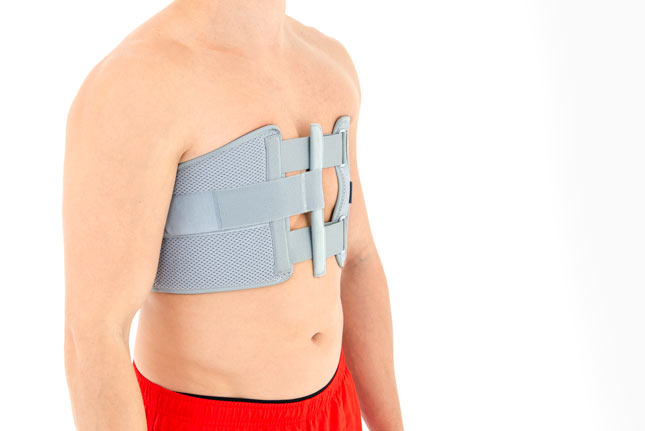
The best solution is to use the Torax belt after chest surgery immediately. The belt stabilizes the breastbone and protects po-op injuries against the torn during sleep of cough. Thanks to using this orthopaedic support, the patient can the following day walk and start cardiological rehabilitation. This solution increase the healing.
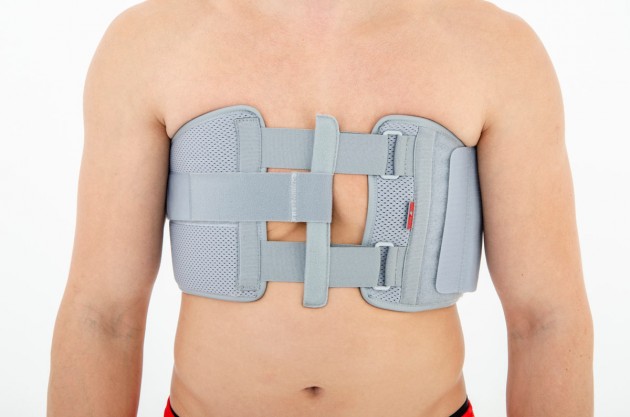
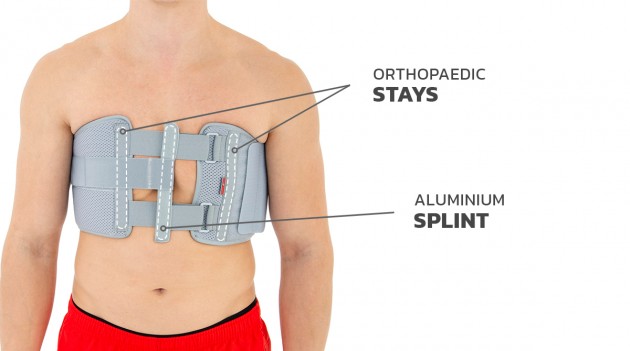
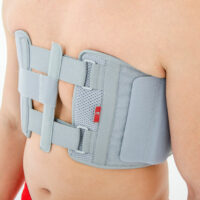
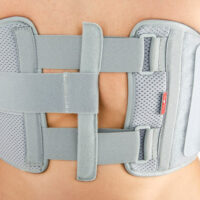
Our Thorax belt AM-TX-02 is the universal device that stabilizes chest and breastbone area precisely. On the front we use system of stabilizing orthopaedic stays and aluminium splint. The belt is made of cotton fabric what allows use it directly onto the skin. Because of the universal size, you can fit it without problem based on cutting the unnecessary part of the belt off. Non-elastic construction, firm polyamide buckles and stable frontal fastening guarantee the best po-op protection against the injury torn.
It is made of comfortable fabric AirDistance II™.
Purpose of use
– after breastbone (sternum) and ribs fractures
– after cariological surgeries
Sizes
| Size | Chest circumference | How to measure |
| Universal | 70-130 cm | 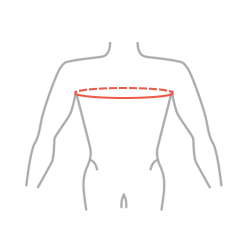 |
Total height of the product: 20 cm (7.9″)
Gallery
Technology
MATERIALS
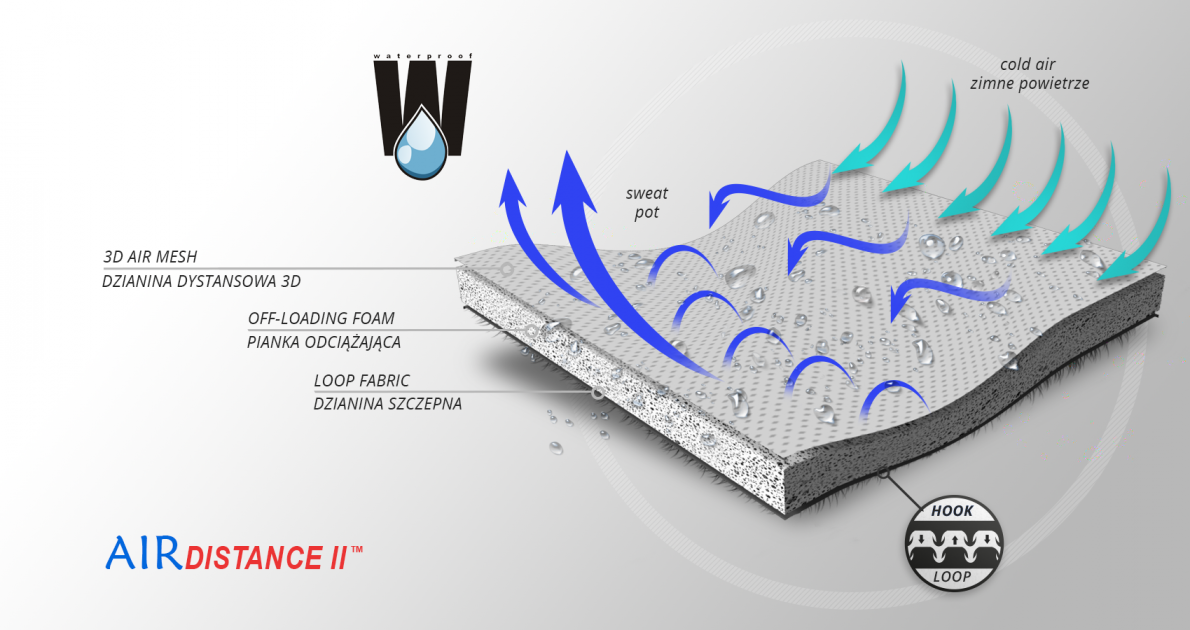
AirDistance II™
AirDistance II™ is an innovative technological fabric. It’s 3-layered lamination, made of 3 parts: durable and self-gripping and polyamide layer, off-loading EVA foam and polyamide 3D spacer material. The external part is used to attach hard components. EVA foam off-loads these splints and the internal 3D spacer layer provides high air permeability and minimal water and moisture absorption. Waterproof material.
STIFFENINGS
Flat aluminum stays
Aluminum stays are made of a special aluminum alloy that guarantees proper stiffness with minimal weight. They have rounded ends to prevent the damage of the product and come in various widths and thicknesses. The level of stabilization of the orthopedic device is defined by proper selection of the width and thickness of the aluminum stays. The stays can be pre-profiled or flat. They do not adapt to the shape of the patient's body, an individual adjustment of the orthosis is required by proper bending of the aluminum stays in the product. Thanks to this function, it is possible to correct the position of the patient's body or the secured joint.

Plastic stays
They come in various widths and thicknesses, are made of various types of plastics, such as polyamide, ABS or acrylic, and these features determine their stiffness. Thanks to their design, they are resistant to water, moisture and sweat. Products equipped with them can be washed without having to remove them from the orthosis. Our plastic stays work only in one direction, perfectly stabilize the laterally protected part of the body, adjusting to it at the same time and have a memory function, thanks to which they always return to their original shape. This function causes the stays in the orthosis to stabilize the swollen limb immediately after the injury and after the swelling has come off. The plastic stays cannot bend and that is why, they cannot correct the body posture or the secured joint.
Setting up
Downloads
ON OUR WEBSITE WE PRESENT MEDICAL DEVICES.
USE THEM ACCORDING TO THE INSTRUCTIONS FOR USE OR LABEL.

 Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
MANUFACTURER / ADVERTISING ENTITY: REH4MAT Sp. z o.o.