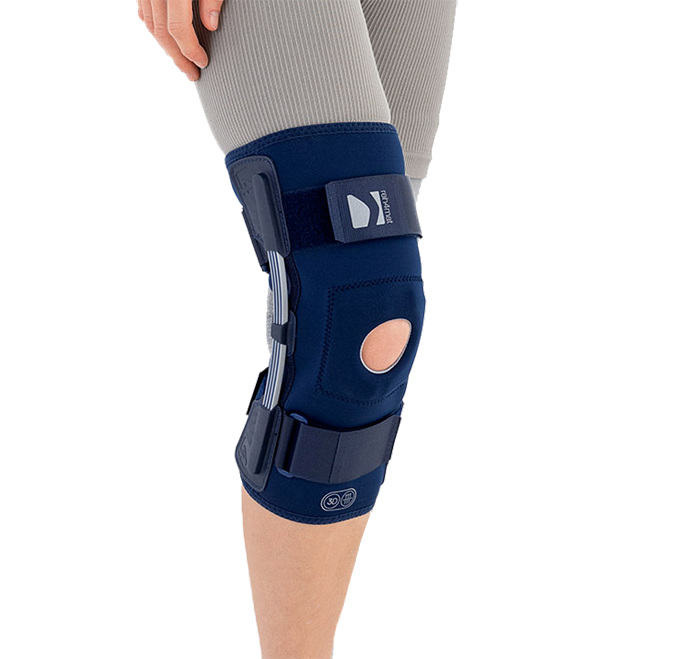
OKD-42 Knee brace
 Lower limb brace
Lower limb brace Breathable
Breathable Class I medical device
Class I medical device Invisible
Invisible Leaf springs
Leaf springsOKD-42 LIGHTWEIGHT KNEE BRACE WITH LEAF SPRING HINGES AND KNEECAP DONUT
Opis
KNEE ARTHROSIS:
Arthrosis, also known as osteoarthritis of the knee, is a chronic condition of the knee in which the cartilage is destroyed, causing pain, stiffness and significant restriction of mobility. The elderly, overweight people, as well as athletes whose knee joint is subjected to heavy loads or physical workers are mainly at risk of knee arthrosis.
Osteoarthritis of the knee is progressive in nature. Unfortunately, there is no effective cure for it, but we distinguish various treatments that can alleviate symptoms and slow its progression. In addition to pharmacology and the use of NSAIDs, i.e. non-steroidal anti-inflammatory drugs, it is also worthwhile to relieve pressure on the knee joint in a professional knee brace of the OKD-42 type.
Characteristics of the product:
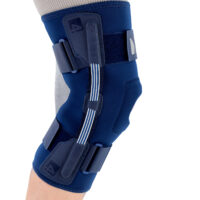
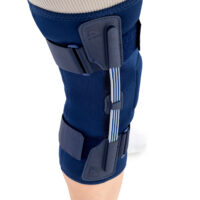
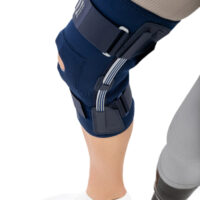
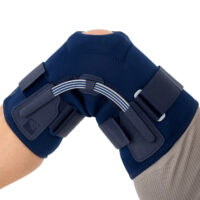
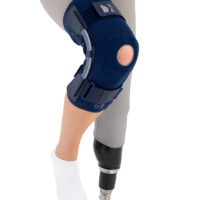
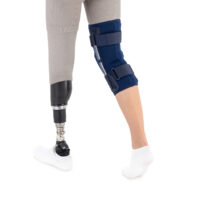
The OKD-42 knee brace is a professional medical device that provides significant support to the knee joint in the medial-lateral direction.
In the device, the knee is supported by leaf spring hinges that act like a lateral shock absorbers that uniquely follow the natural rotational movement of the knee. The leaf spring hinges also relieve pressure on the meniscus, reducing pressure on the joint and reducing wear and tear on the articular cartilage.
The OKD-42 knee brace acts like a knee, without restricting the movement of extension and flexion.
Made of skin-friendly, breathable 3D knit fabric, it ensures full oxygenation of the limb during increased physical activity.
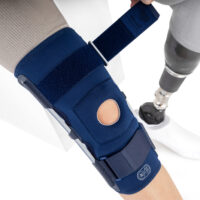
The OKD-24 knee brace also features an elastic patellar donut to ensure alignment and proper mobility. This solution reduces pain in the anterior compartment of the joint and prevents dislocation or chondromalacia of the patella.
The kneecap donut has a dynamic design, perfectly adapting to any shape of the limb, ensuring its maintenance in the joint axis and individual fit.
The anatomical, lightweight design of the product keeps the shock absorbers in the lateral axis of the knee joint, providing proper support and pressure relief to the joint.
The low-profile design of the product allows it to be worn freely under clothing.
Purpose of use:
- Osteoarthritis and instability of the knee.
- Rheumatoid conditions
- ACL, MCL or LCL ligament injury.
- Po-op follow-up treatment
- Knee osteoarthrosis (OA)
Sizes
| Size | Knee circumference | How to measure |
| S | 30 – 34 cm |  |
| M | 34 – 38 cm | |
| L | 38 – 42 cm | |
| XL | 42 – 46 cm | |
| 2XL | 46 – 50 cm |
Fits for both knees.
Total length of the product:
S – 2XL: 33 cm
Gallery
Technology
MATERIALS
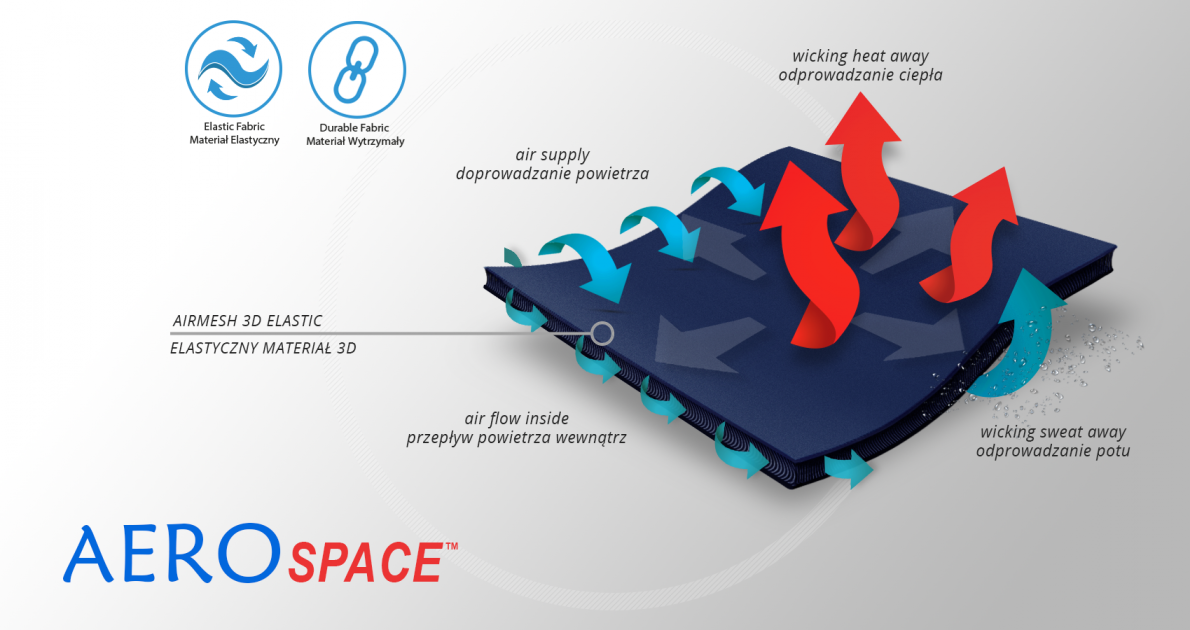
AeroSpace™
AeroSpace™ is an innovative new generation raw material. It is a distance elastic 3D knitted fabric consisting of two layers of facings and an interlacing that creates the appropriate thickness of the raw material and has relieving properties. The knitwear is made of the highest quality polyester yarn - guaranteeing high mechanical strength and spandex ensuring its proper flexibility. This material is characterized by a very low weight, high flexibility and a very large openwork structure, allowing for very easy drainage of sweat from the body and bringing fresh air to the skin. Products made of this raw material are neutral to the secured joint, do not heat or cool it, but ensure its proper compression and fit and reduce muscle vibrations generated during physical exertion. Its thickness and 3D structure perfectly relieves the orthopedic splints, stays or other elements mounted on the product and guarantees velvety softness to the touch.
STIFFENINGS
Leaf spring hinges
Leaf spring hinges are made of high quality alloy of metal and plastic. This construction provides anatomic knee motion with excellent lateral knee stabilization.

Setting up
Downloads
ON OUR WEBSITE WE PRESENT MEDICAL DEVICES.
USE THEM ACCORDING TO THE INSTRUCTIONS FOR USE OR LABEL.

 Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
MANUFACTURER / ADVERTISING ENTITY: REH4MAT Sp. z o.o.