Knee support EB-P/RZ
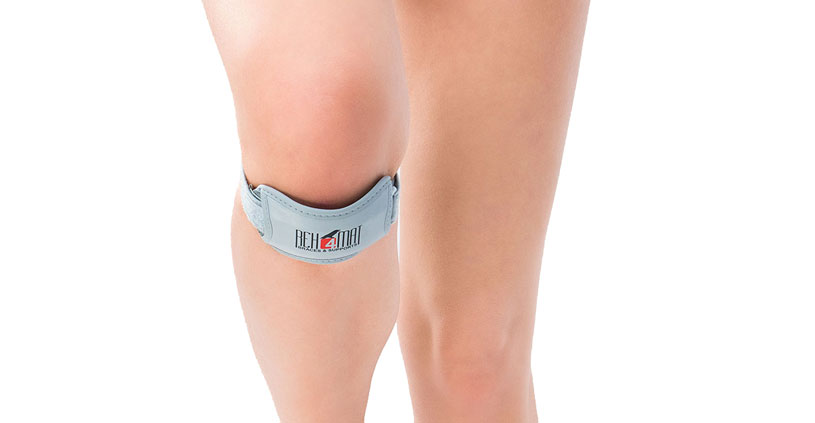

 Knee brace
Knee brace Class I medical device
Class I medical device Double-sided
Double-sided Invisible
Invisible Minimum device - maximum effect
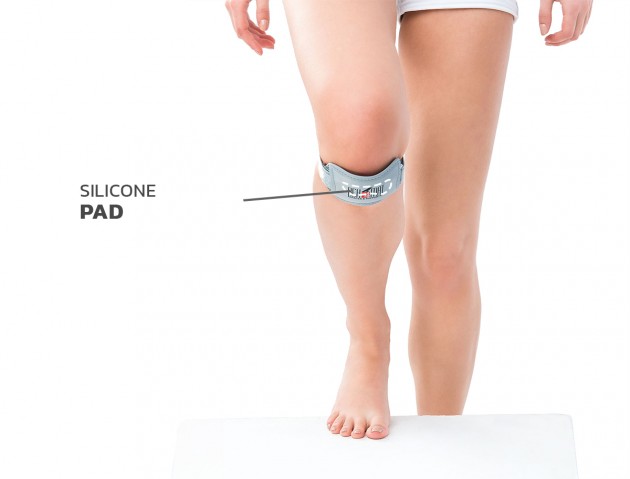
Minimum device - maximum effect Silicone pad
Silicone pad Skin-friendly
Skin-friendly Universal size
Universal sizeJUMPER’S KNEE STRAP
Description
Jumper’s knee brace EB-P/RZ was made of the best materials. The brace support the external insertion of the quadriceps femoris tendon. Thanks to that it stabilizes and support the knee-cap and prevents the patellofemoral pain. The brace contains silicon anatomic-shaped pad also, which is semi-rigid and provides great knee support. Furthermore, this anatomic shape prevents slipping the brace during the physical activities. Additionally the use of Velcro tape allows for final fit of the support to the knee joint. This is the best solution for athletes. Made of AirDistance II™ and Sanmed II™.
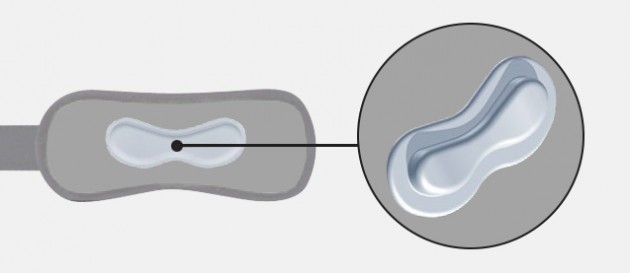
Silicon pad
Purpose of use
- Osgood-Schlattera Disease
- Jumpers knee (Patellar tendinitis)
- Stressed patellar ligament
- Patellarfemoral joint degeneration (chondromalacia patellae)
- Subluxation/proximal hypermobility of the tibiofibular joint
MiniMax principle

MiniMax is the first principle of REH4MAT that focuses on maximum effect in minimum device. Designing of our braces, we focus heavily on their effectiveness where, aside from functionality, the size of braces is significant. Unique constructions of our devices provide maximum safety and protection with little sizes. It allows to use comfortably our braces in sport shoes or under clothes.
Sizes
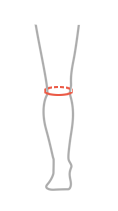
| Size | Circumference under the knee | How to measure |
| Universal size, fits left and right leg | min 26 cm – max 42cm (min 10,2″ – max 16,5″) |
 |
Total height of the product: 5 cm (2″)
Setting up
Gallery
Technology
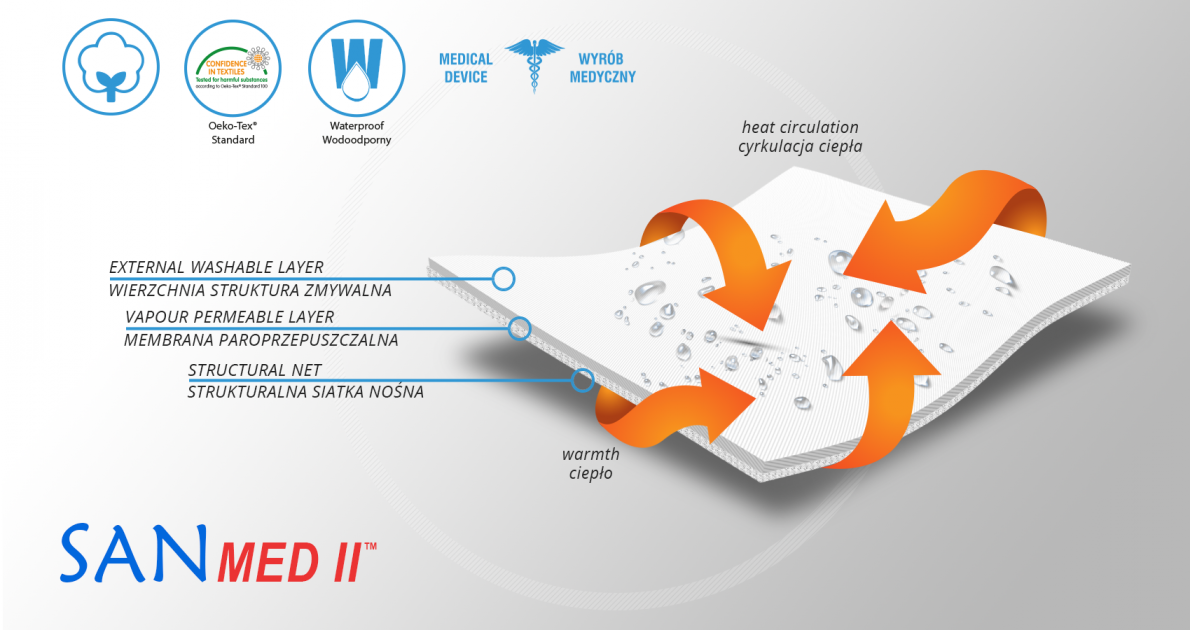
MATERIALS
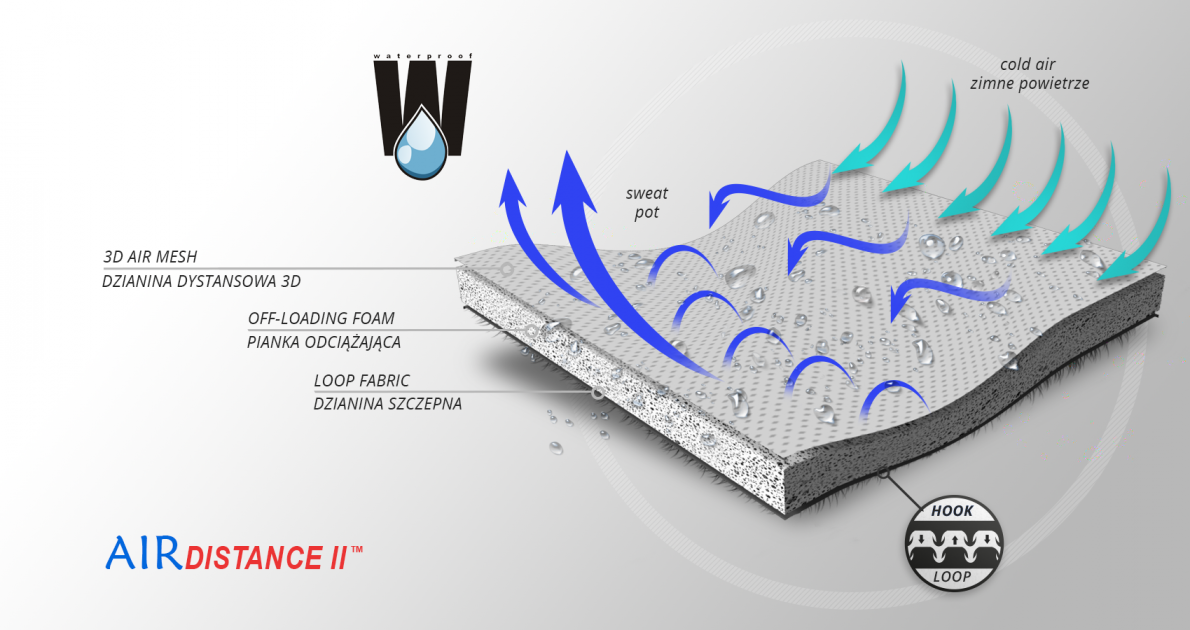
AirDistance II™
AirDistance II™ is an innovative technological fabric. It’s 3-layered lamination, made of 3 parts: durable and self-gripping and polyamide layer, off-loading EVA foam and polyamide 3D spacer material. The external part is used to attach hard components. EVA foam off-loads these splints and the internal 3D spacer layer provides high air permeability and minimal water and moisture absorption. Waterproof material.
Sanmed II™
Sanmed II™ is an innovative and very friendly for human skin fabric. It can be sterilized so it can be used by a large number of patients. This material is lightweight and easy to form. What’s the most important, Sanmed II™ can be used in places with a high risk of infections because it consists of antibacterial components based on silver zeolite (AgZ). It provides long-term efficiency and prevents even the most infectious bacteria such as MRSA or E.coli. Sanmed C463 meets the condition according to the Health Minister`s ordinance of 3 November 2004 and Council Directive 93/42/EWG of 14 June 1993. An additional asset of the Sanmed II™ is vapour permeability and water resistance. That’s why it is used as a cover of waterproof anti-bedsore hospital mattresses and couches. In addition, our fabric holds Oeko-Tex Standard 100 certification what proves its chemical neutrality for human skin. Waterproof material.
PADDINGS
ACL ligaments silicone pads
Silicone pads in natural color that aim is to relieve the ACL ligaments. These pads have an anatomical shape and are made of silicone with the appropriate hardness and elasticity, guaranteeing the proper therapeutic effect.
Setting up
Downloads
ON OUR WEBSITE WE PRESENT MEDICAL DEVICES.
USE THEM ACCORDING TO THE INSTRUCTIONS FOR USE OR LABEL.

 Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
MANUFACTURER / ADVERTISING ENTITY: REH4MAT Sp. z o.o.