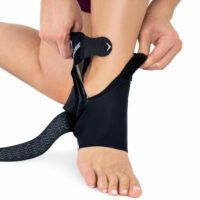
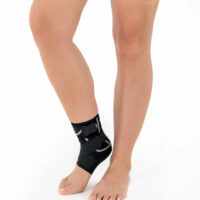
Ankle support EB-SS BLACK MELANGE


 Ankle brace
Ankle brace Breathable
Breathable Class I medical device
Class I medical device Compression
Compression Figure-8 strap
Figure-8 strap Skin-friendly
Skin-friendlyANKLE BRACE
Description
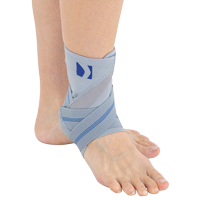
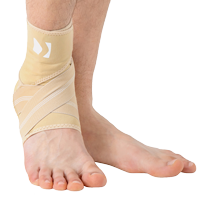
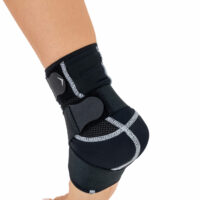
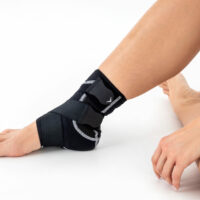
Ankle support EB-SS is made of ProFit™ fabric. The second used material is AirRubber III™, which has unidirectional elasticity.
Properties
Stabilization and warming up of the ankle joint, reduction of pain, improvement of movement ability and quality. The support is made of material which provides elastic pressure to the foot during its movement which helps reduce post-injury swelling and plays prevention role in sport training. The application and conditions of use depend on the decision of the leading doctor.
Purpose of use
- after ankle joint injuries such as strain
- as an support after fractures and surgeries
- ankle instability
- ankle degeneration
- prophylactically in physical activities
Sizes
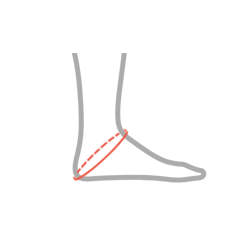
| Size | Heel circumference | How to measure |
| S | 27 – 30 cm (10,6″-11,8″) |
 |
| M | 30 – 33 cm (11,8″-13″) |
|
| L | 33 – 36 cm (13″-14,2″) |
|
| XL | 36 – 39 cm (14,2″-15,4″) |
Right and left ankle specific.
Total height of the product: 18 cm (7,1″)
Colors
Gallery
Technology
MATERIALS
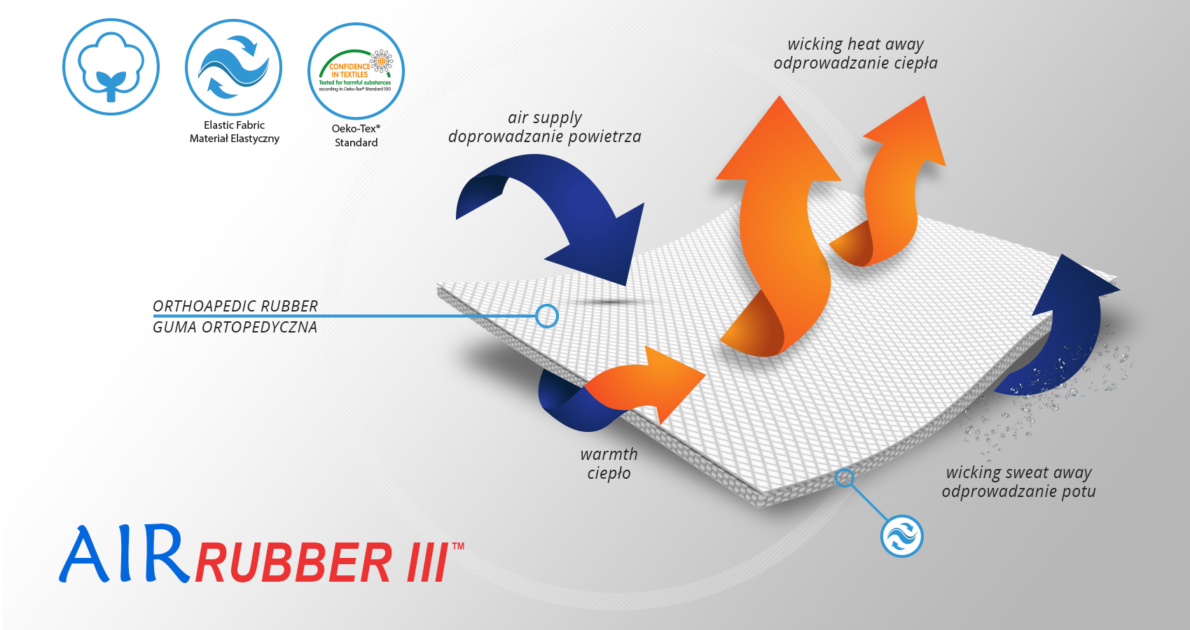
AirRubber III™
AirRubber III™ has unidirectional elasticity. It can be stretched, increasing the length, not width, what improves compression. Between the braids air flows freely and skin can breathe. In addition in high compression, these rubber braids provide a gentle massage for skin. Our orthopedic rubber is very friendly for skin.
Setting up
Downloads
ON OUR WEBSITE WE PRESENT MEDICAL DEVICES.
USE THEM ACCORDING TO THE INSTRUCTIONS FOR USE OR LABEL.

 Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
MANUFACTURER / ADVERTISING ENTITY: REH4MAT Sp. z o.o.