Torso support ENDURO-02


 Breathable
Breathable Class I medical device
Class I medical device Innovative
Innovative Latex-free
Latex-free Skin-friendly
Skin-friendlyENDURO 2
SACRO-LUMBAR SUPPORT – HIGH KIDNEY BELT
Description

The kidneys are delicate organs that require attention. The diseases such as inflammation, which occurs as a result of chilling, for long periods without developing symptoms. Inflammation requires careful treatment. Breakage leads to the recurrence of the disease. Negligence of the treatment can cause threatening kidney complications and even irreversible damages required dialysis or a transplant. Inflammation can affect different parts of the organ – glomeruli, pelvis and parenchyma. Kidney Belt is an item of clothing that should have every motorcyclist.
Product description
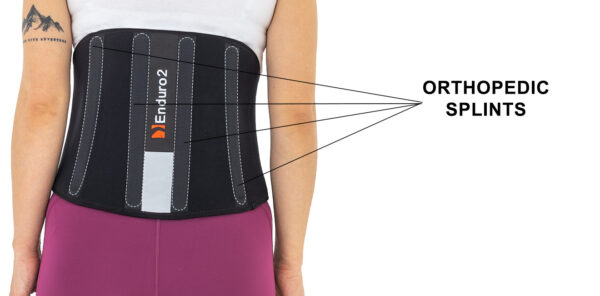
In addition, in the rear part of the device occur four orthopedic stays, which stabilize lumbar spine.
What’s more, in the rear part is fluorescence element, which make the biker more visible during night.

Our kidney belt is elegant and „predatory” but delicate embroidery makes it charming.
Sizes
| Size | Waist circumference | How to measure |
|---|---|---|
| S | 65-75 cm(25,6″-29,5″) | 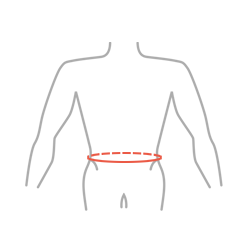 |
| M | 75-85 cm(29,5″-33,5″) | |
| L | 85-97 cm(33,5″-38,2″) | |
| XL | 97-110 cm(38,2″-43,3″) | |
| 2XL | 110-125 cm(43,3″-49,2″) | |
| 3XL | 125-145 cm(49,2″-57,1″) |
Overall height of the brace
Front: 16 cm
Rear: 28 cm
Gallery
Technology
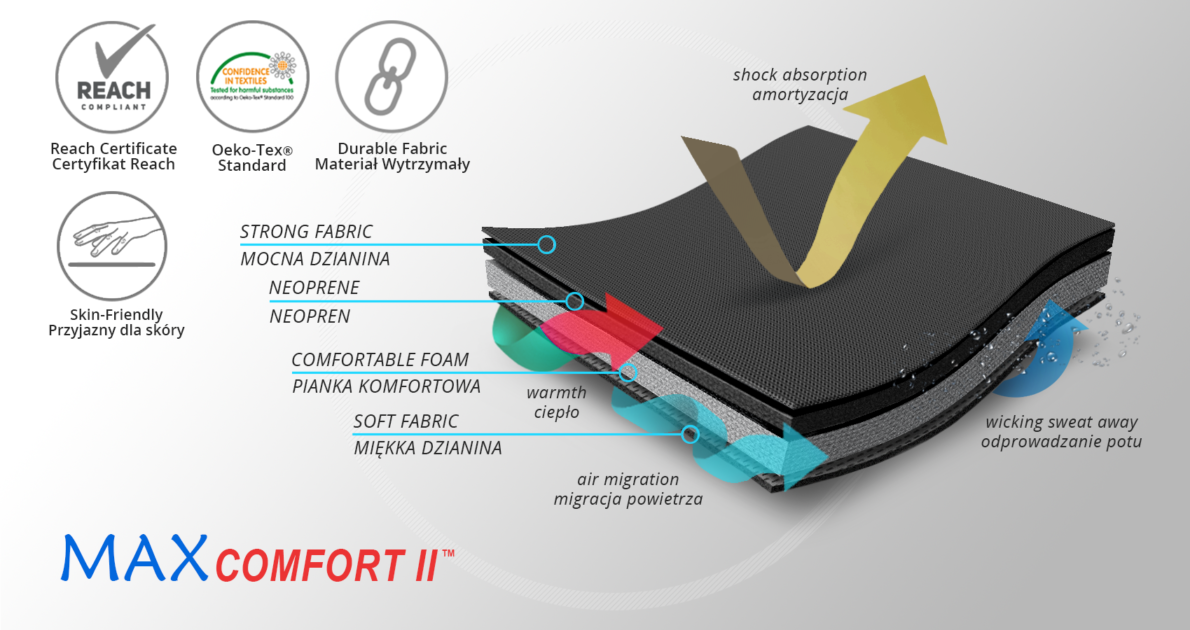
MATERIALS
MaxComfort II™
MaxComfort II™ is a new generation multi-layer material with excellent relieving function. The use of a product made of MaxComfort II™ means excellent stabilization and very high comfort of use. From the side of the body there is a soft, skin-friendly fabric with OekoTex Standard 100 approval, which has perforations that facilitate air flow and ensure skin ventilation. The next layer is soft foam, which ensures the comfort of using the orthosis, eliminates irregularity between the orthosis and the body, increases the contact surface of the orthosis with the patient's skin, which reduces local pressure on the body and has an anti-bedsore effect. The next layer is neoprene foam, which task is to relieve the structural elements of the orthosis from the patient's body. The last layer is a strong knitted fabric, the structure of which guarantees high durability and wear resistance.
STIFFENINGS
Orthopedic stays from ABS
Orthopedic stays available in various width, thickness and shapes are cut from ABS boards. Their role is not to stiffen the orthosis in order to support a joint or other part of the body, but to prevent wrinkling and uncontrolled movement of the fabrics of the orthosis./p>

Setting up
Downloads
Accessories
ACCESSORIES / PRODUCTS TO BE USED WITH
ON OUR WEBSITE WE PRESENT MEDICAL DEVICES.
USE THEM ACCORDING TO THE INSTRUCTIONS FOR USE OR LABEL.

 Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
MANUFACTURER / ADVERTISING ENTITY: REH4MAT Sp. z o.o.