Wrist brace AM-OSN-U-12


 Thumb brace
Thumb brace Class I medical device
Class I medical device ER
ER Innovative
Innovative Minimum device - maximum effect
Minimum device - maximum effectSkier’s Thumb Brace for UCL Ligament Injury
Description
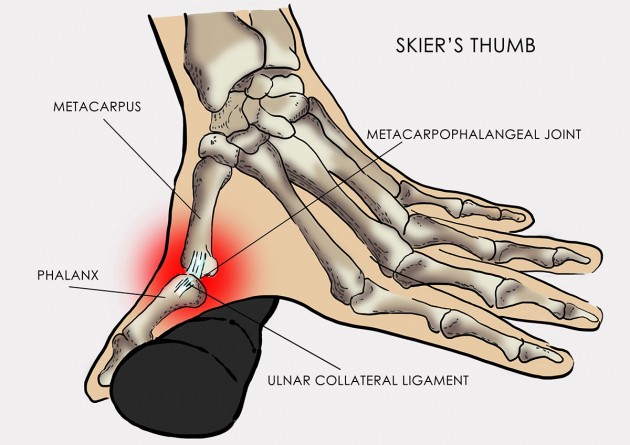
Skier’s thumb
Skier’s thumb, also known as Gamekeeper’s thumb, is an injury to the ulnar collateral ligament (UCL) of the thumb. It’s the most common sport injury of hand. UCL injury makes up 8%-10% of all skiing accidents. Pathomechanism of this condition bases on involuntary abduction and hyperextension of the first digit of the hand.
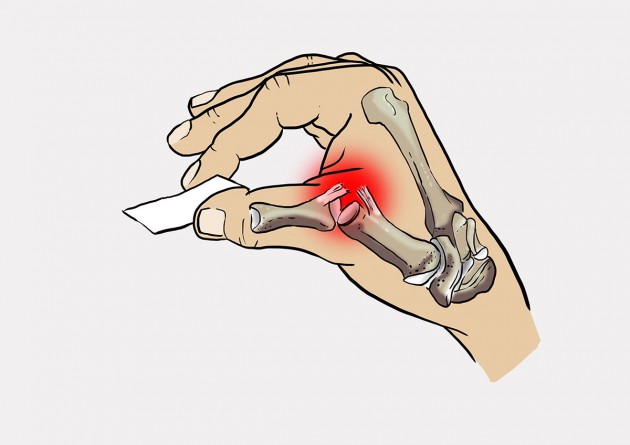
Patient with skier’s thumb feels pain in case of pincer grip.
Total torn UCL causes pain during pincer grip.
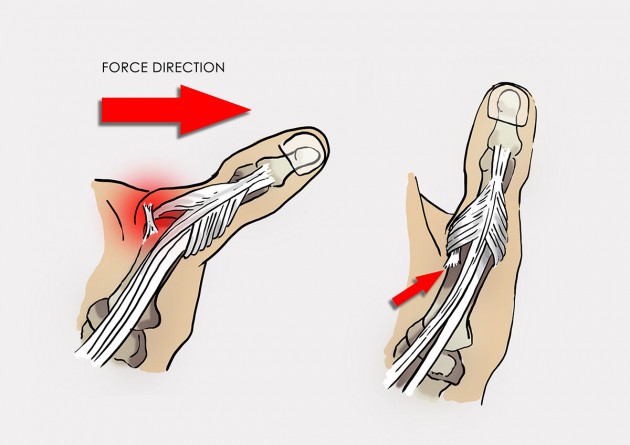
Total torn UCL is called also Stener lesion and it occurs when the aponeurosis of the adductor pollicis muscle becomes interposed between the ruptured ulnar collateral ligament (UCL) of the thumb and its site of insertion at the base of the proximal phalanx. This injury required surgery for treatment.
Mechanism of Stener lesion.
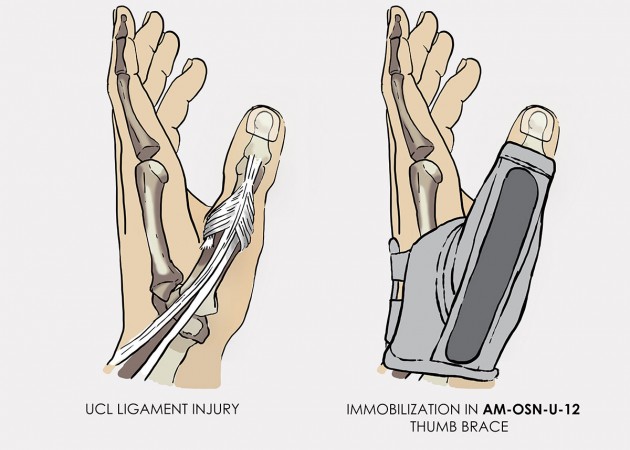
To avoid such complicated and dangerous injury, you should use our hand brace AM-OSN-U-12, dedicated for skier’s thumb.
Product’s description
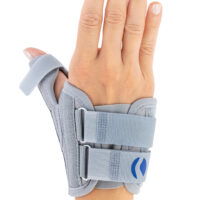
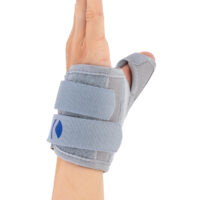
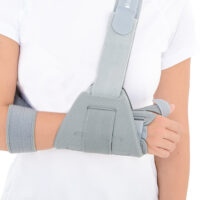
Special construction allows to use our brace under ski glove. You can use it prophylactically or as an post-injury thumb immobilizer. The brace’s small size doesn’t immobilize the wrist joint, so you can use it every day.
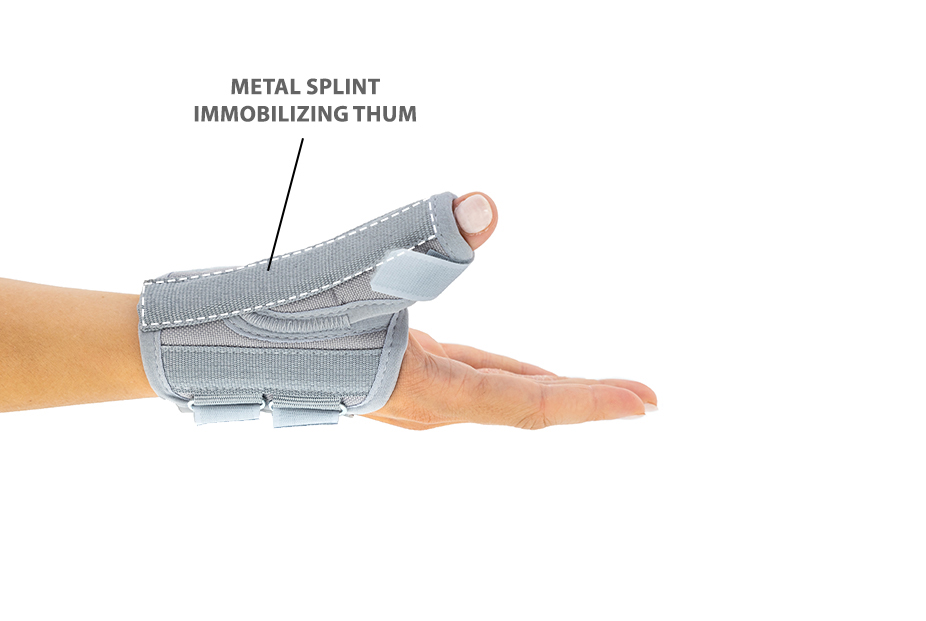
Additionally, the thumb brace is equipped with metal splint immobilizing the thumb.
The brace is very easy to putting on and Velcro straps provide excellent fitting and compression. You can also cut the excessive Velcro strap off.
Metal splint stabilizes the metacarpophalangeal joint and reduces the risk of sudden thumb abduction and hyperextension.
Purpose of use
- Skier’s Thumb – injury of the ulnar collateral ligament (UCL)
- instability of the metacarpophalangeal joint of the thumb in the early osteoarthritis
- joint dislocation
- after osteotomy, arthroplasty or ligament reconstruction
- Benett or Roland fracture
- twisted CMC joint
- thumb muscles strain
- prophylactically in sport
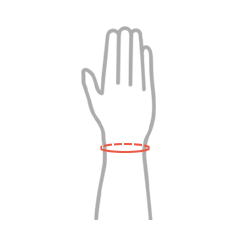
Sizes
| Size | Wrist circumference | How to measure |
| S | 13-15 cm (5,1″-5,9″) |
 |
| M | 15-17 cm (6,1″-6,7″) |
|
| L | 17-19 cm (6,9″-7,5″) |
|
| XL | 19-21 cm (7,7″-8,3″) |
Left / right side available
Total height of the product: 10 cm (3,9″)
Gallery
Technology
MATERIALS
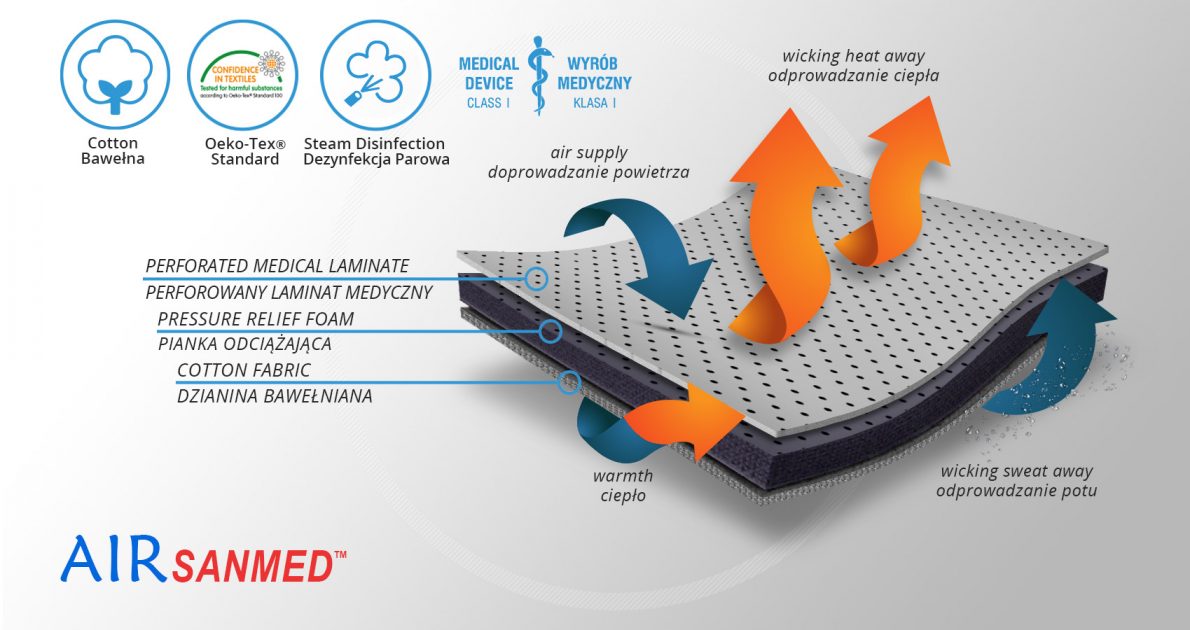
AirSanmed™
AirSanmed™ isn’t elastic what provides excellent stabilization. The skin has contact with cotton terry with Oeko-Tex Standard 100 certificate. There is semi-rigid perforated foam EVA inside that protect the skin against the metal splints influence. External side of the fabric is perforated medical laminate with antibacterial properties of Silver Zeolite. It provides long-term efficacy and prevents the most dangerous infectious microorganisms such as MRSA and E.coli. AirSanmed™ is in accordance with Health Minister`s ordinance of 3 November 2004 and Council Directive 93/42/EWG of 14 June 1993.
STIFFENINGS
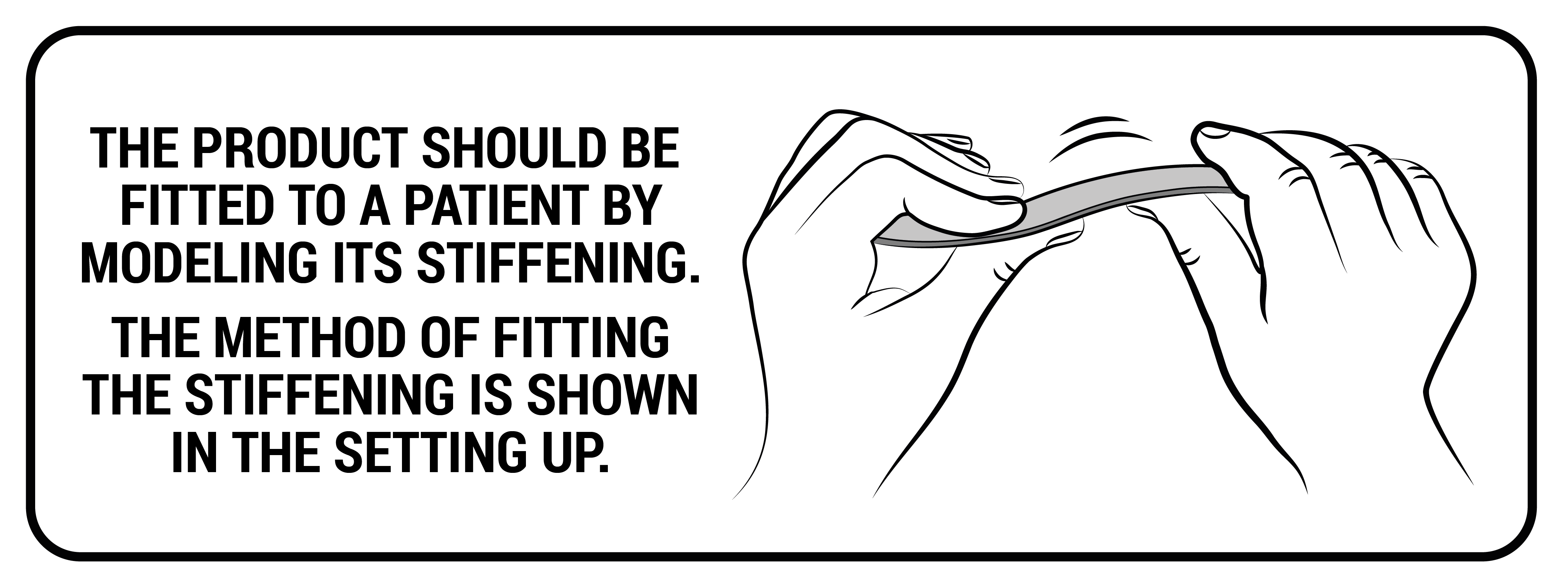
Profiled aluminum stays
These are splints and orthopedic stays of various thickness and width, which are made of various types of aluminum alloys. All these splints and stays, before mounting to a given orthosis, have been pre-profiled, which allows for fitting the product to the body of a specific patient. However, for the correct operation of the device, they should be precisely bent to the patient's body by an orthopedist, physiotherapist or orthopedist technician. Only this action guarantees the proper protection and support of the patient's body.

Setting up
Downloads
ON OUR WEBSITE WE PRESENT MEDICAL DEVICES.
USE THEM ACCORDING TO THE INSTRUCTIONS FOR USE OR LABEL.

 Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
MANUFACTURER / ADVERTISING ENTITY: REH4MAT Sp. z o.o.