Wrist support AM-OSN-U-05


 Wrist brace
Wrist brace Anatomic wrist splint
Anatomic wrist splint Breathable
Breathable Cast replacement
Cast replacement Chemical free
Chemical free Class I medical device
Class I medical device Cotton
Cotton Anatomic patella donut
Anatomic patella donut Double-sided
Double-sided ER
ER Latex-free
Latex-free Recommended by specialists
Recommended by specialists Skin-friendly
Skin-friendly Zespół cieśni nadgarstka
Zespół cieśni nadgarstkaWRIST AND FOREARM BRACE
Description
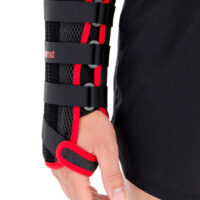
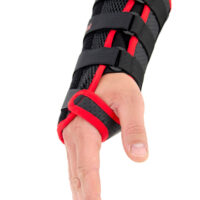
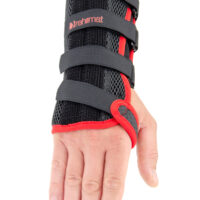
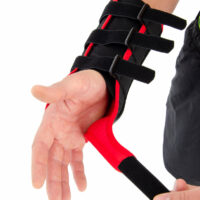
The wrist support is provided with anatomically shaped, malleable and removable palm strip with sweat-resistant powder coating applied.
The palmar part of orthosis and forearm is equipped with soft and elastic pad which makes the product more comfortable. The structure and used materials help to relieve carpal tunnel syndrome.
Dorsal part of orthosis is equipped with 2 plastic stays. The Palm Strip and 2 dorsal stays form circumferential three-points compression allowing adequate support and unloading of radiocarpal joint.
The fitting on the Wrist by three velcro closures as well as hand strap. Whole range of adjustment guarantees easy fitting and uniform stabilization of the hand.

3 points stabilization
L3P (LOCK 3 POINTS)
Wrist support is made of innovative materials ActiveSpace™ and AirSanmed™.
Purpose of use
- after wrist injuries
- bursitis
- joint degeneration or inflammation
Sizes
| Size | Wrist circumference | How to measure |
| S | 13-15 cm (5,1″-5,9″) |
 |
| M | 15-17 cm (5,9″-6,7″) |
|
| L | 17-19 cm (6,7″-7,5″) |
|
| XL | 19-21 cm (7,5″-8,3″) |
Left/right side available.
Total length of the product: 23 cm (9,1″)
Gallery
Technology
MATERIALS
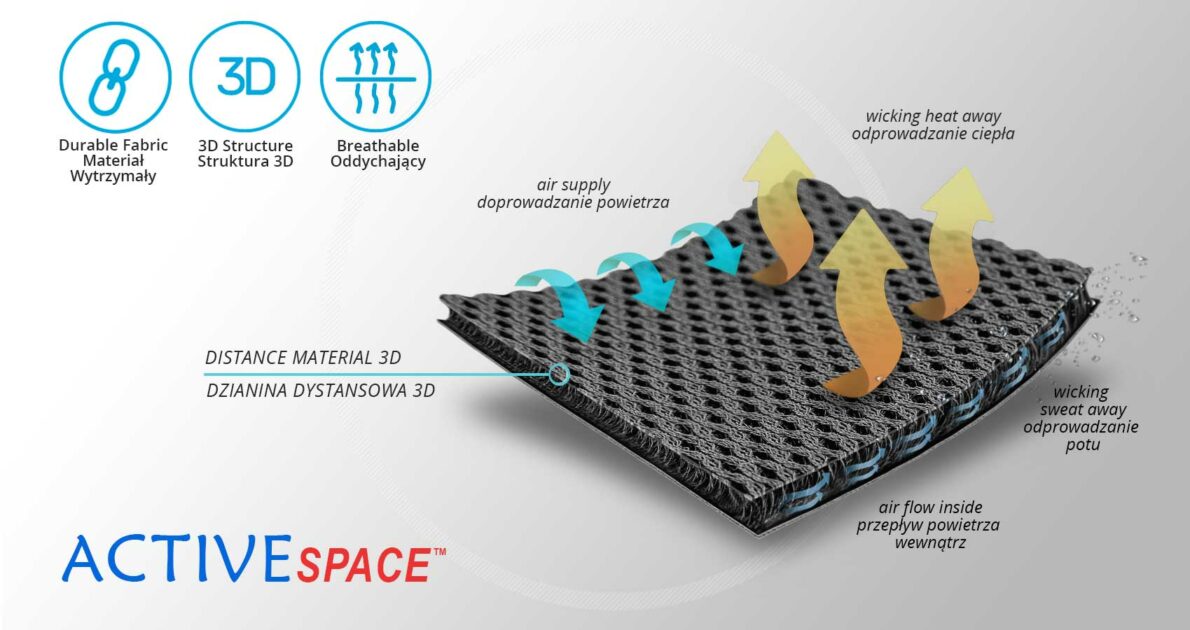
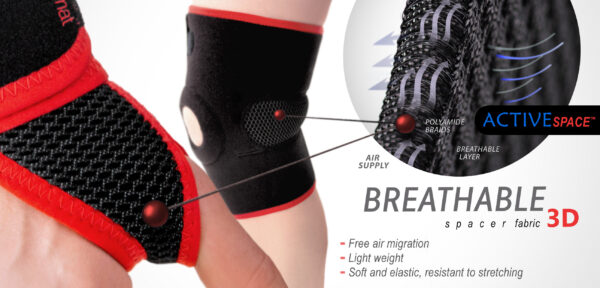
ActiveSpace™
ActiveSpace™ is a spacer, polyamide 3D lamination with high skin ventilation. It is very lightweight, consisted of 2 layers. Between them, we use polyamide braids. ActiveSpace™ is not elastic what improves stabilization. Inside the lamination, between 2 layers, the air flows freely, maintaining minimal water and moisture absorption. Waterproof material.
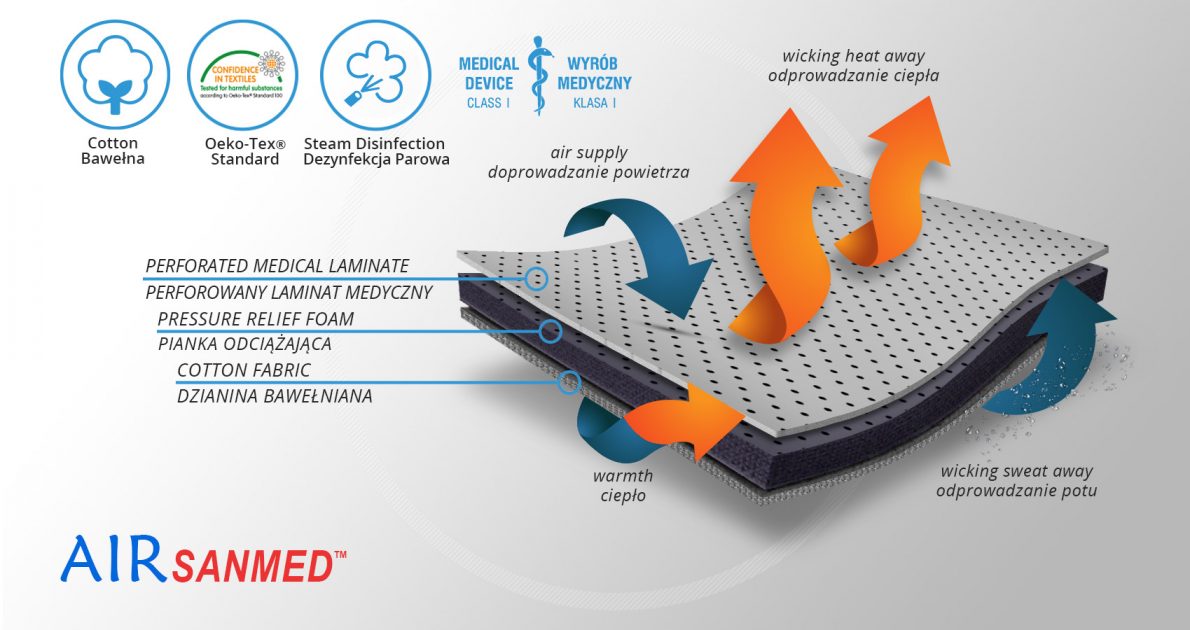
AirSanmed™
AirSanmed™ isn’t elastic what provides excellent stabilization. The skin has contact with cotton terry with Oeko-Tex Standard 100 certificate. There is semi-rigid perforated foam EVA inside that protect the skin against the metal splints influence. External side of the fabric is perforated medical laminate with antibacterial properties of Silver Zeolite. It provides long-term efficacy and prevents the most dangerous infectious microorganisms such as MRSA and E.coli. AirSanmed™ is in accordance with Health Minister`s ordinance of 3 November 2004 and Council Directive 93/42/EWG of 14 June 1993.
TECHNOLOGICAL SYSTEMS
System for Carpal Tunnel Syndrome - CTSyndrome
 Carpal tunnel syndrome is the most common entrapment neuropathy encountered in clinical practice. It is caused by compression of the median nerve as it passed through the carpal canal at the wrist. The most common causes of compression of the median nerve at this location include flexor tenosynovitis, rheumatoid arthritis, pregnancy, amyloidosis and poor wrist position.
Carpal tunnel syndrome presents as pain, numbness, paresthesias, and associated weakness in the hand and wrist that radiate to the thumb, index finger, middle finger and ring finger.
Carpal tunnel syndrome is the most common entrapment neuropathy encountered in clinical practice. It is caused by compression of the median nerve as it passed through the carpal canal at the wrist. The most common causes of compression of the median nerve at this location include flexor tenosynovitis, rheumatoid arthritis, pregnancy, amyloidosis and poor wrist position.
Carpal tunnel syndrome presents as pain, numbness, paresthesias, and associated weakness in the hand and wrist that radiate to the thumb, index finger, middle finger and ring finger.
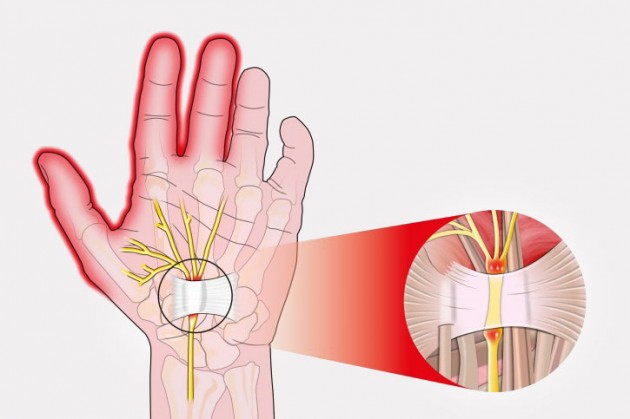 The best treatment for carpal tunnel syndrome is using wrist splint brace.
However, many patients aren’t aware that ordinary simple wrist splint worsens their carpal tunnel syndrome! Directly compression by splint exacerbates problem and pain. Furthermore, patient without entrapment neuropathy, using the wrist brace due to bones fracture, can suffer from carpal tunnel syndrome after many weeks of immobilization and wrist compression.
Because of that, using the special wrist splint brace, reducing the carpal canal pressure is essential.
REH4MAT introduced to mostly wrist braces innovative solution CTSyndrome. This is special pad on wrist splint which protects carpal canal against the excessive compression. Due to that fact, electrical impulses in median nerve go freely.
The best treatment for carpal tunnel syndrome is using wrist splint brace.
However, many patients aren’t aware that ordinary simple wrist splint worsens their carpal tunnel syndrome! Directly compression by splint exacerbates problem and pain. Furthermore, patient without entrapment neuropathy, using the wrist brace due to bones fracture, can suffer from carpal tunnel syndrome after many weeks of immobilization and wrist compression.
Because of that, using the special wrist splint brace, reducing the carpal canal pressure is essential.
REH4MAT introduced to mostly wrist braces innovative solution CTSyndrome. This is special pad on wrist splint which protects carpal canal against the excessive compression. Due to that fact, electrical impulses in median nerve go freely.
STIFFENINGS
Palm strip
Plastic stays
They come in various widths and thicknesses, are made of various types of plastics, such as polyamide, ABS or acrylic, and these features determine their stiffness. Thanks to their design, they are resistant to water, moisture and sweat. Products equipped with them can be washed without having to remove them from the orthosis. Our plastic stays work only in one direction, perfectly stabilize the laterally protected part of the body, adjusting to it at the same time and have a memory function, thanks to which they always return to their original shape. This function causes the stays in the orthosis to stabilize the swollen limb immediately after the injury and after the swelling has come off. The plastic stays cannot bend and that is why, they cannot correct the body posture or the secured joint.
Setting up
Downloads
Accessories
ON OUR WEBSITE WE PRESENT MEDICAL DEVICES.
USE THEM ACCORDING TO THE INSTRUCTIONS FOR USE OR LABEL.

 Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
MANUFACTURER / ADVERTISING ENTITY: REH4MAT Sp. z o.o.