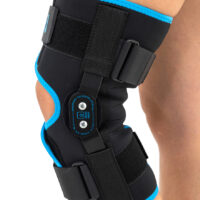
Lower-extremity support AM-OSK-O/2
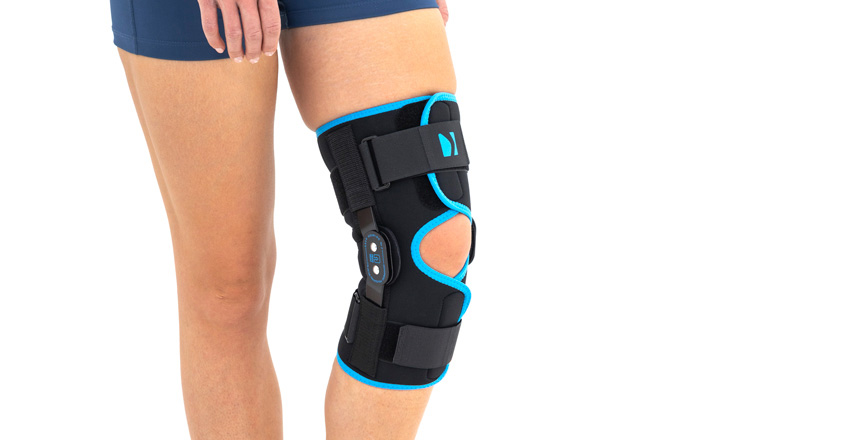

 Knee brace
Knee brace Breathable
Breathable Double-sided
Double-sided Polycentric joint 2
Polycentric joint 2 Skin-friendly
Skin-friendlyOPEN KNEE BRACE WITH POLYCENTRIC HINGES
Description
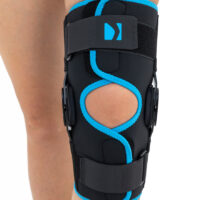
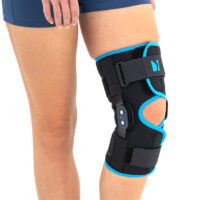
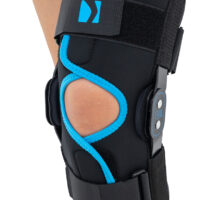
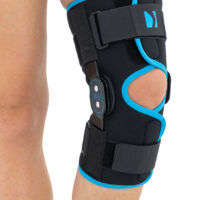
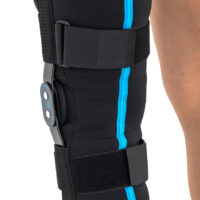
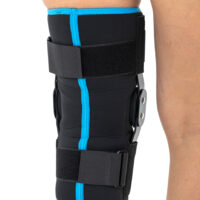
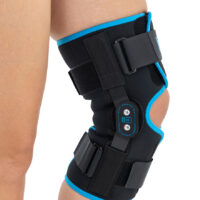
Wrap around knee brace AM-OSK-O/2 is the professional medical device 1st class, which improves the gait and the rehabilitation process. It is made of innovative ActivePren™️ fabric.
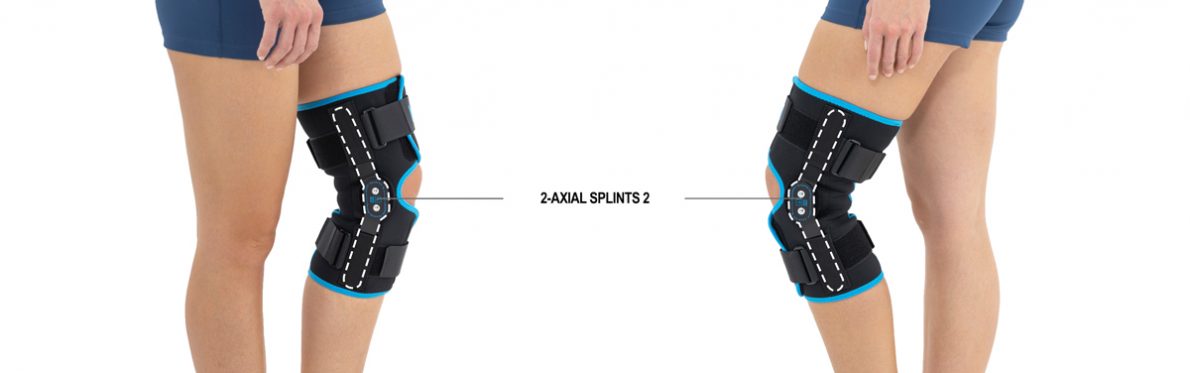
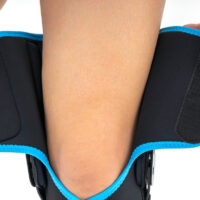
Thanks to the use of the AirSupply System the protected joint is well aired and excessive humidity is removed from the skin. The device is equipped with independent VELCRO straps and 2-axial aluminium sides splints without ROM adjustment but stabilizing the knee joint in the frontal plane. By maintaining the stable temperature and elastic compression, the brace has analgesic properties and reduces effusions and swelling after injuries. Its wrap-around construction is the best solution for seniors with limited mobility and for people, who have disproportion between thigh and calf.
Purpose of use
AM-OSK-O/2 was created for seniors suffered from chronic knee instability with limited mobility. It should be used in case of:
- after injury of the knee which didn’t require surgery (dislocation with damage of ligaments, muscle sprains and strains)
- in chronic knee instability (frontal and sagittal)
- in postoperative rehabilitation
- joint degeneration and/or knee instability due to injury, illness in case of patients not qualified or refusing surgery treatment
Sizes
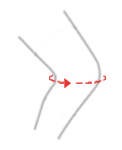
| Size | Knee circumference | How to measure |
| S | 30 – 34 cm (11,8″-13,4″) |
 |
| M | 34 – 38 cm (13,4″-15″) |
|
| L | 38 – 42 cm (15″-16,5″) |
|
| XL | 42 – 46 cm (16,5″-18,1″) |
|
| 2XL | 46 – 50 cm (18,1″-19,7″) |
|
| 3XL | 50 – 55 cm (19,7″-21,7″) |
|
| 4XL | 55 – 59 cm (21,7″-23,2″) |
Fits for both knees.
Total length of the product: 32 cm (12,6″)
Some of the sizes are available while stocks last: S
Gallery
Technology
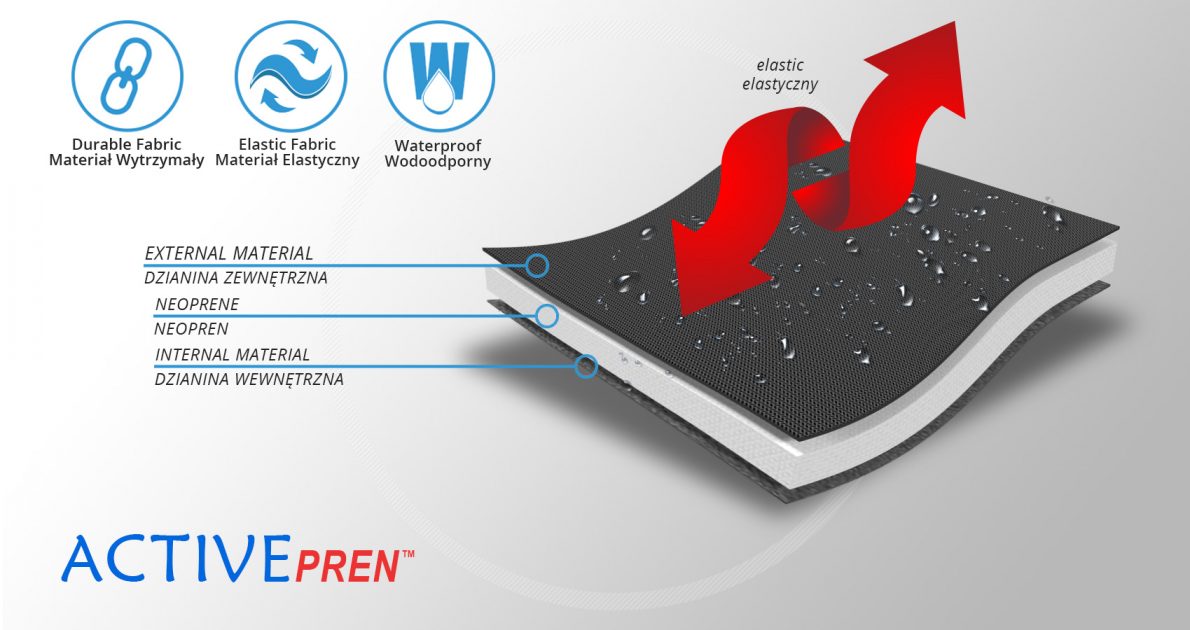
MATERIALS
ActivePren™
ActivePren™ is an active three-layer material consisting of two elastic jersey cover fabrics and a core made of neoprene foam. This material is characterized by softness and high flexibility. A very important advantage of this material is the fact that it is not a knitted product, it does not have thick fibers, so that the weaves of the material do not imprint on the patient's skin and do not cause abrasionsin places of high compression. Products made of ActivePren are the strongest and most effective stabilizing orthoses available on the market.
STIFFENINGS
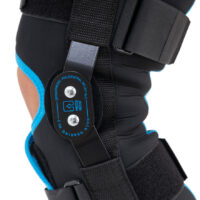
Splint 2
Splints [2] – double-axis polycentric splints equipped with rack and pinion, which reflects the anatomic movement of the knee joint. It is made of high quality aluminium alloy, with durable sanded cover, which protects the splint from influence of sweat and salt. Splint is equipped with specially shaped silicone insert to stabilize the splint and additionally release stress from the knee joint.

PADDINGS
3D supports
3D relief supports are independent technical solutions to relieve the rigid elements of a given orthosis. These elements are made of supporting foams or EVA foam. These foams are connected with various types of skin-friendly materials and materials with an adhesive function. These pads have the appropriate shape and color adapted to the type of orthosis. They relieve both metal elements of orthoses, such as splints, stays, underwires and orthopedic drop locks, as well as other elements that should not come into direct contact with the patient's skin. These pads have an anatomical shape and are made of comfortable foam with proper hardness and elasticity, guaranteeing the proper therapeutic effect.
Setting up
Downloads
Accessories
ACCESSORIES / PRODUCTS TO BE USED WITH
ON OUR WEBSITE WE PRESENT MEDICAL DEVICES.
USE THEM ACCORDING TO THE INSTRUCTIONS FOR USE OR LABEL.

 Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
MANUFACTURER / ADVERTISING ENTITY: REH4MAT Sp. z o.o.