Lower limb support EB-SK/2RA BEIGE
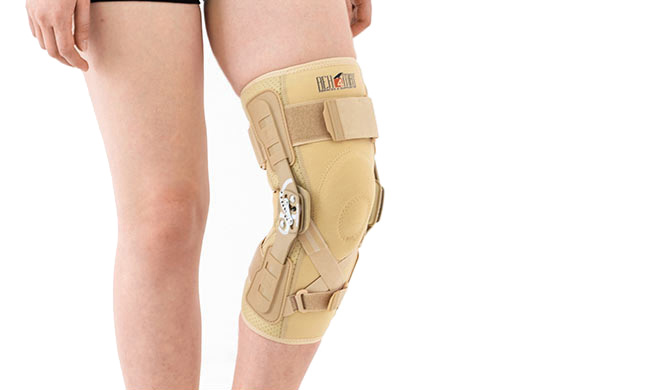

 Knee brace
Knee brace Anatomic joint 2RA
Anatomic joint 2RA Class I medical device
Class I medical device Compression
Compression Double-sided
Double-sided Innovative
Innovative Invisible
Invisible Latex-free
Latex-free Skin-friendly
Skin-friendlyKNEE BRACE WITH ANATOMICAL ROM ADJUSTMENT AND ACL SUPPORT
Description
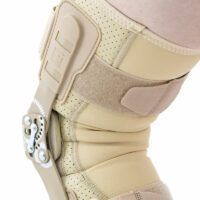
EB-SK/2RA knee brace is closed construction professional medical device, which increases the gait quality and is the support in rehabilitation of the locomotor system. It stabilizes knee joint and support the muscles insertions. It is made of a structural material ProFit™ and Active3D™. Rubber bands made of AirRubber II™ material.
Purpose of use
- the ligaments injuries (ACL, PCL, LCL, MCL)
- knee sprain or strain
- knee dislocation
- after non-operative knee injuries (ligaments or muscles insertions strains)
- in joint degenerations and instability caused by sudden injuries or other conditions
- prophylactically in sport
It is possible to use a thinner pad under the drop lock.
Sizes
| Size | Knee circumference | How to measure |
| S | 30-34 cm (11,8″-13,4″) |
 |
| M | 34-38 cm (13,6″-15″) |
|
| L | 38-42 cm (15,2″-16,5″) |
|
| XL | 42-46 cm (16,7″-18,1″) |
|
| XXL | 46-50 cm (18,3″-19,7″) |
Total length of product: 36 cm (14,2″)
Colors
Gallery
Technology
MATERIALS
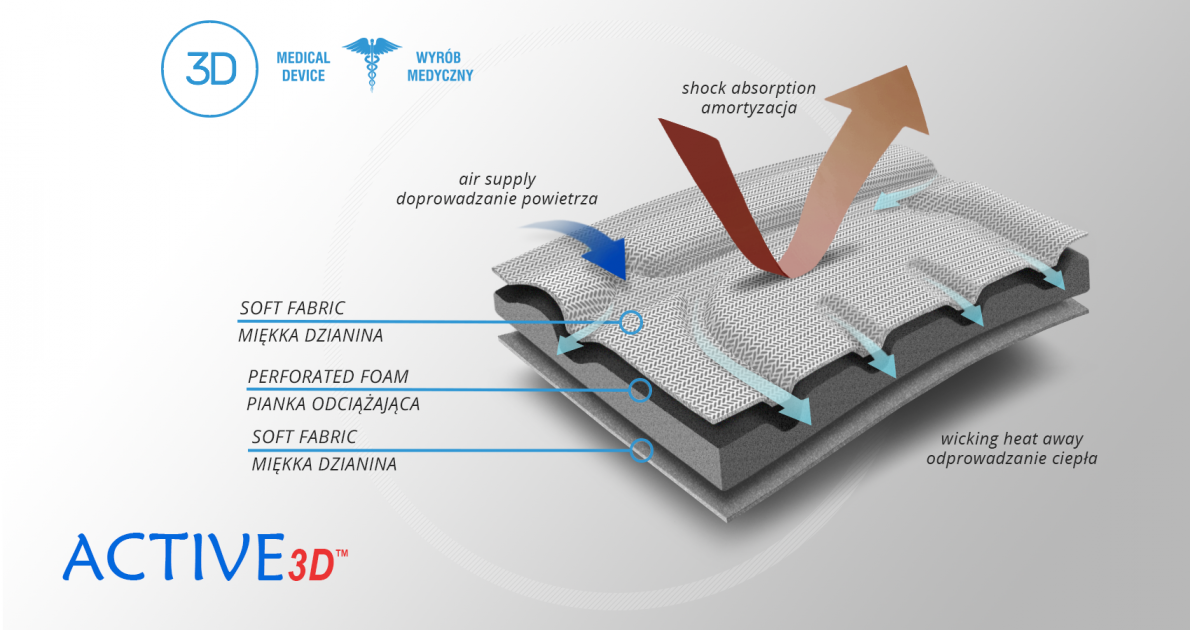
Active3D™
Active3D™ is thermoformed fabric made of special foamed, cell-closed designed material. It reduces the pressure on the body or any abrasions made by orthopaedic stays and aluminum splints. It is fully waterproof fabric and does not absorb sweat. It’s easy to clean. Due to its features, the fabric is an excellent product for making medical orthopaedic braces and orthoses. ACTIVE 3D™ has various external self-gripping layers. Our material has special, thermoformed properties and may be shaped according to the functional goals of the final braces.
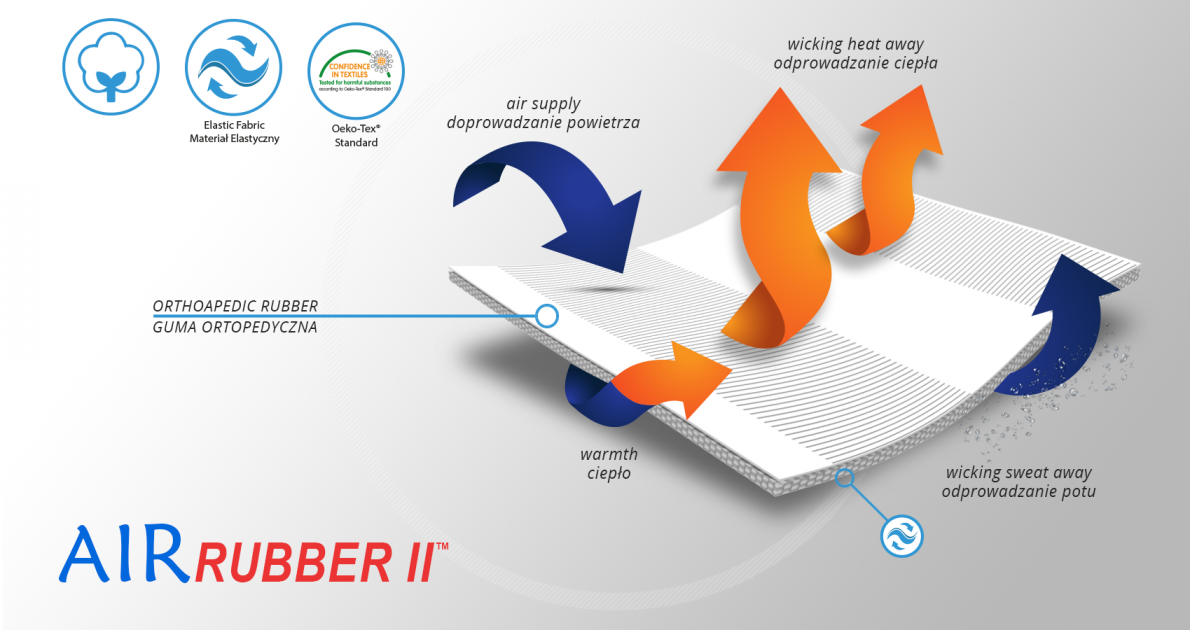
AirRubber II™
AirRubber II™ is breathable, perforated and has unidirectional elasticity. It can be stretched, increasing the length, not width, what improves compression. Between the braids air flows freely and skin can breathe. In addition in high compression, these rubber braids provide a gentle massage for skin. Our orthopedic rubber is very friendly for skin.
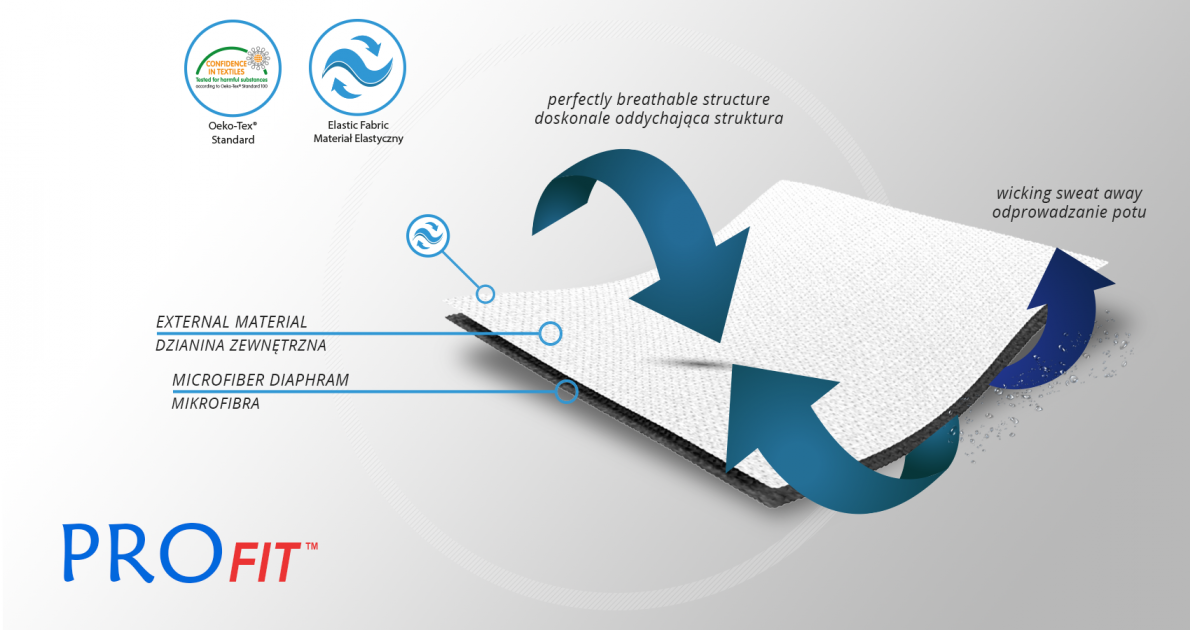
ProFit™
ProFit™ is a double-layer fabric that is elastic in all directions and fits to limb precisely. The external side is made of spandex providing excellent compression and absorbs muscle shocks in physical activity. The internal side consists of microfibre, cotton and elastane what makes it skin-friendly, breathable and provides velvet softness. ProFit™ is friendly and neutral for the skin and has Oeko-Tex Standard 100 certificate. In some products this material can have an internal elastic cotton layer.
STIFFENINGS
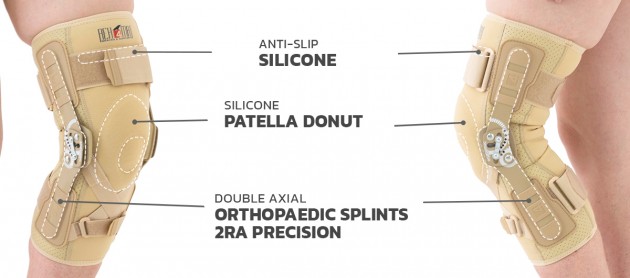
Splint 2RA Precision
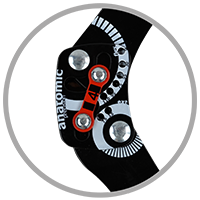 Splints 2RA Precision are a double axis anatomic splints which reflect the knee movement and during performance do not change their position towards the joint axis. Splints 2RA are double axis polycentric splints, with coupling mechanism with two main conductive elements which move of parabolic tracks. Such splints are mainly used in all knee joint braces and, apart from angle adjustment, the high lateral knee stabilization is very important. The splints are manufactured with high quality of sanded aluminium, durable cover which what makes splints neutral to the influence of sweat and salt. Flexion and extension angle adjustment is possible with special allen screws. Every brace is equipped with special Allen key which is necessary for adjustment. Anatomic splints provides hyperextension block, 30°, 55°, 75°, 95°, 110° flexion and 15° or 75° extension. The angle adjustment clock is secured from manipulation by unauthorized people. Standard length of the splints is: 290 and 390 mm. The splint and its design are patented in European Union by Reh4Mat company.
Splints 2RA Precision are a double axis anatomic splints which reflect the knee movement and during performance do not change their position towards the joint axis. Splints 2RA are double axis polycentric splints, with coupling mechanism with two main conductive elements which move of parabolic tracks. Such splints are mainly used in all knee joint braces and, apart from angle adjustment, the high lateral knee stabilization is very important. The splints are manufactured with high quality of sanded aluminium, durable cover which what makes splints neutral to the influence of sweat and salt. Flexion and extension angle adjustment is possible with special allen screws. Every brace is equipped with special Allen key which is necessary for adjustment. Anatomic splints provides hyperextension block, 30°, 55°, 75°, 95°, 110° flexion and 15° or 75° extension. The angle adjustment clock is secured from manipulation by unauthorized people. Standard length of the splints is: 290 and 390 mm. The splint and its design are patented in European Union by Reh4Mat company.
PADDINGS
3D supports
3D relief supports are independent technical solutions to relieve the rigid elements of a given orthosis. These elements are made of supporting foams or EVA foam. These foams are connected with various types of skin-friendly materials and materials with an adhesive function. These pads have the appropriate shape and color adapted to the type of orthosis. They relieve both metal elements of orthoses, such as splints, stays, underwires and orthopedic drop locks, as well as other elements that should not come into direct contact with the patient's skin. These pads have an anatomical shape and are made of comfortable foam with proper hardness and elasticity, guaranteeing the proper therapeutic effect.
Patella silicone support
A whole series of silicone supports made in various shapes and colors to relieve the patella or to stabilize it. These pads have appropriate shapes and protrusions, thanks to which the orthosis perfectly fulfills its function. These pads have an anatomical shape and are made of silicone with the appropriate hardness and elasticity, guaranteeing the proper therapeutic effect.
Setting up
Downloads
Accessories
ACCESSORIES / PRODUCTS TO BE USED WITH
ON OUR WEBSITE WE PRESENT MEDICAL DEVICES.
USE THEM ACCORDING TO THE INSTRUCTIONS FOR USE OR LABEL.

 Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
MANUFACTURER / ADVERTISING ENTITY: REH4MAT Sp. z o.o.