Groins brace AM-2PP


 Groin brace
Groin brace Hernia belt
Hernia belt Chemical free
Chemical free Class I medical device
Class I medical device Double-sided
Double-sided Ecological
Ecological Hypoallergenic
Hypoallergenic Invisible
Invisible Latex-free
Latex-free Skin-friendly
Skin-friendlyDOUBLE HERNIA BELT
Description
Hernia
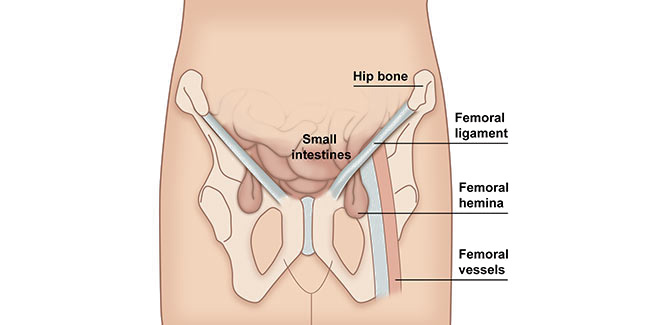
Hernia is a serious condition because can causes intestinal necrosis. Every internal organ has own place and function. The abdominal wall (muscles, ligaments) maintain them in the required position providing their proper working. The intraabdominal pressure increases during laughing, coughing or eliminating. In this situation the muscles work more creating the abdominal press. If some part of the abdominal wall is weaker, it starts delaminating and ruptures making the celoschisis where the bowels can cram. In this situation the hernia occurs.
Product description
In this case, when the surgery can wait, the AM-2PP hernia belt is the best solution.
The belt is made of elastic, friendly for skin material AirRubber III™ and is equipped with anatomic inguinal pelottes.
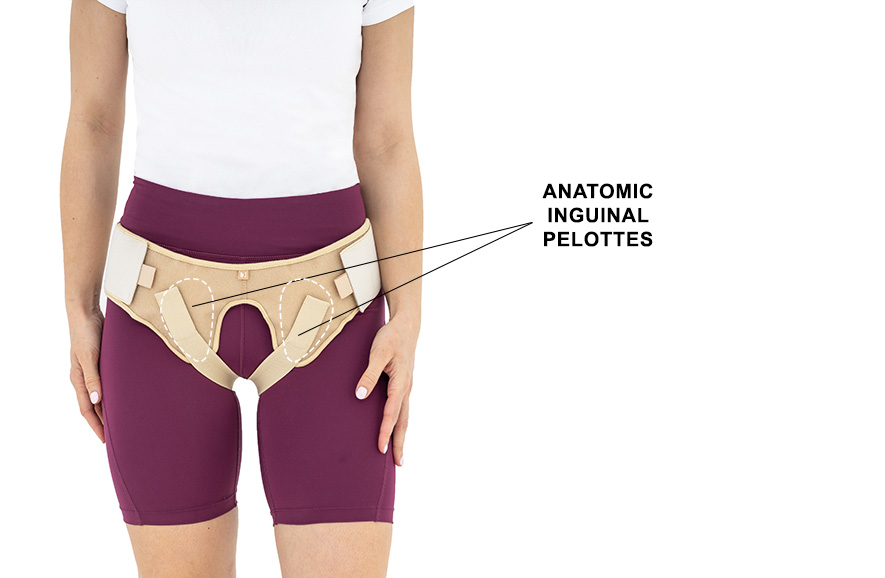
In addition, the belt holds inguinal strap which stabilizes it on the body precisely. The hernia belt can be used by women and men.
Purpose of use
- limits existing inguinal hernias (right and left)
- as an prevention the groins after surgeries when the muscles and ligaments are weaker
- chronic supports existing small both (right and left) inguinal hernias when the patient is not operable
Not use in case of irreducible hernias, because the inguinal pelotte can cause intestinal wall impairment!
Sizes
| Size | Hip circumference at the widest point | How to measure |
|---|---|---|
| S | 86 – 94 cm (33,9″ – 37″) |
 |
| M | 94 – 102 cm (37″ – 40,2″) |
|
| L | 102 – 110 cm (40,2″ – 43,3″) |
|
| XL | 110 – 125 cm (43,3″ – 49,2″) |
|
| 2XL | 125 – 140 cm (49,2″ – 55,1″) |
Total height of the product:
front: 14 cm (5.5″)
rear: 8 cm (3,15″)
Gallery
Technology
MATERIALS
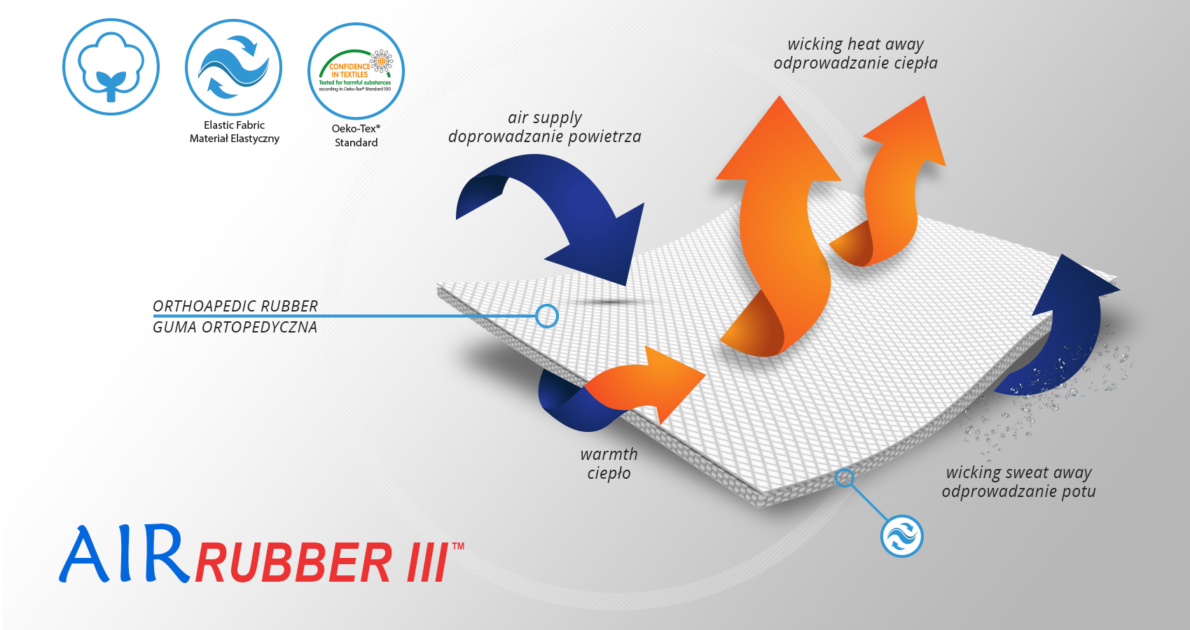
AirRubber III™
AirRubber III™ has unidirectional elasticity. It can be stretched, increasing the length, not width, what improves compression. Between the braids air flows freely and skin can breathe. In addition in high compression, these rubber braids provide a gentle massage for skin. Our orthopedic rubber is very friendly for skin.
CottonComfort™
CottonComfort™ is one of the most comfortable, antiallergic and ecological orthopedic fabric that we use in the production of our products. This raw material consists of a very comfortable polyurethane foam, laminated on one or both sides with a soft and comfortable cotton knit. The knit is made of natural cotton, without any chemicals containing formaldehyde. It is certified by Oeko-Tex® Standard 100, which guarantees safety to the patient's skin. The lamination process takes place without the use of any kinds of glue, but with the use of the flame method, which changes the surface of the polyurethane foam into a sticky surface to which the outer facings of the raw material stick. Thanks to the use of such a production technology, we do not unnecessarily introduce chemical chemicals into the structure of the raw material and we ensure a very high strength of such a connection. CottonComfort™ is used in orthoses in the most delicate places, so as not to cause skin irritation and so that the soft structure of the material tightly fills the free spaces between the orthosis and the patient's body.
PADDINGS
Hernia pads
Hernia pads in the shape of hemispheres of various diameters, made of natural-colored silicone. These pads have an anatomical shape and are made of silicone with the appropriate hardness and elasticity, guaranteeing non-invasive and full filling of the abdominal hernia opening.
Setting up
Downloads
ON OUR WEBSITE WE PRESENT MEDICAL DEVICES.
USE THEM ACCORDING TO THE INSTRUCTIONS FOR USE OR LABEL.

 Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
Class I medical device in accordance with Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017. on medical devices.
MANUFACTURER / ADVERTISING ENTITY: REH4MAT Sp. z o.o.